NDC 70165-300
Cotempla XR-ODT
Methylphenidate
Cotempla XR-ODT is a Oral Tablet, Orally Disintegrating in the Human Prescription Drug category. It is labeled and distributed by Neos Therapeutics, Lp. The primary component is Methylphenidate.
| Product ID | 70165-300_7dec6513-60e0-42e3-890d-dedee031c696 |
| NDC | 70165-300 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Cotempla XR-ODT |
| Generic Name | Methylphenidate |
| Dosage Form | Tablet, Orally Disintegrating |
| Route of Administration | ORAL |
| Marketing Start Date | 2017-06-20 |
| Marketing Category | NDA / NDA |
| Application Number | NDA205489 |
| Labeler Name | Neos Therapeutics, LP |
| Substance Name | METHYLPHENIDATE |
| Active Ingredient Strength | 26 mg/1 |
| Pharm Classes | Central Nervous System Stimulant [EPC],Central Nervous System Stimulation [PE] |
| DEA Schedule | CII |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 70165-300-30
5 BLISTER PACK in 1 CARTON (70165-300-30) > 6 TABLET, ORALLY DISINTEGRATING in 1 BLISTER PACK
| Marketing Start Date | 2017-06-20 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 70165-300-30 [70165030030]
Cotempla XR-ODT TABLET, ORALLY DISINTEGRATING
| Marketing Category | NDA |
| Application Number | NDA205489 |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Billing Unit | EA |
| Marketing Start Date | 2017-06-20 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| METHYLPHENIDATE | 25.9 mg/1 |
OpenFDA Data
| SPL SET ID: | 33f70f58-c871-42c8-8adb-345caeafefcd |
| Manufacturer | |
| UNII | |
| RxNorm Concept Unique ID - RxCUI |
Pharmacological Class
- Central Nervous System Stimulant [EPC]
- Central Nervous System Stimulation [PE]
Medicade Reported Pricing
70165030030 COTEMPLA XR-ODT 25.9 MG TABLET
Pricing Unit: EA | Drug Type:
NDC Crossover Matching brand name "Cotempla XR-ODT" or generic name "Methylphenidate"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 70165-100 | Cotempla XR-ODT | Methylphenidate |
| 70165-200 | Cotempla XR-ODT | Cotempla XR-ODT |
| 70165-300 | Cotempla XR-ODT | Cotempla XR-ODT |
| 68968-5552 | Daytrana | methylphenidate |
| 68968-5553 | Daytrana | methylphenidate |
| 68968-5554 | Daytrana | methylphenidate |
| 68968-5555 | Daytrana | methylphenidate |
| 0378-8260 | Methylphenidate | Methylphenidate |
| 0378-8261 | Methylphenidate | Methylphenidate |
| 0378-8262 | Methylphenidate | Methylphenidate |
| 0378-8263 | Methylphenidate | Methylphenidate |
| 0781-2361 | Methylphenidate | methylphenidate |
| 0781-2362 | Methylphenidate | methylphenidate |
| 0781-2363 | Methylphenidate | methylphenidate |
| 0781-2364 | Methylphenidate | methylphenidate |
Trademark Results [Cotempla XR-ODT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
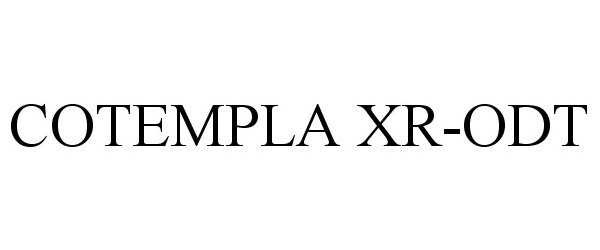 COTEMPLA XR-ODT 86847780 5387222 Live/Registered |
Neos Therapeutics, Inc. 2015-12-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.