NDC 70857-002
Traulevium
Belladonna, Arnica Montana Radix, Aconitum Napellus, Chamomilla, Symphytum Officinale, Calendula Officinalis, Hamamelis Virginiana, Millefolium, Hepar Sulphuris Calcareum, Mercurius Solubilis, Hypericum Perforatum, Bellis Perennis, Echinacea Angustifolia, Echinacea Purpurea.
Traulevium is a Oral Tablet, Chewable in the Human Otc Drug category. It is labeled and distributed by Medical Technology Products, Inc.. The primary component is Atropa Belladonna; Arnica Montana; Aconitum Napellus; Matricaria Recutita; Comfrey Root; Calendula Officinalis Flowering Top; Hamamelis Virginiana Root Bark/stem Bark; Achillea Millefolium; Calcium Sulfide; Mercurius Solubilis; Hypericum Perforatum; Bellis Perennis; Echinacea Angustifolia; Echinacea Purpurea.
| Product ID | 70857-002_a4fee32e-3ddd-4ff5-a110-3a65ad2d59a2 |
| NDC | 70857-002 |
| Product Type | Human Otc Drug |
| Proprietary Name | Traulevium |
| Generic Name | Belladonna, Arnica Montana Radix, Aconitum Napellus, Chamomilla, Symphytum Officinale, Calendula Officinalis, Hamamelis Virginiana, Millefolium, Hepar Sulphuris Calcareum, Mercurius Solubilis, Hypericum Perforatum, Bellis Perennis, Echinacea Angustifolia, Echinacea Purpurea. |
| Dosage Form | Tablet, Chewable |
| Route of Administration | ORAL |
| Marketing Start Date | 2016-10-03 |
| Marketing Category | UNAPPROVED HOMEOPATHIC / UNAPPROVED HOMEOPATHIC |
| Labeler Name | Medical Technology Products, Inc. |
| Substance Name | ATROPA BELLADONNA; ARNICA MONTANA; ACONITUM NAPELLUS; MATRICARIA RECUTITA; COMFREY ROOT; CALENDULA OFFICINALIS FLOWERING TOP; HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK; ACHILLEA MILLEFOLIUM; CALCIUM SULFIDE; MERCURIUS SOLUBILIS; HYPERICUM PERFORATUM; BELLIS PERENNIS; ECHINACEA ANGUSTIFOLIA; ECHINACEA PURPUREA |
| Active Ingredient Strength | 4 [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1; [hp_X]/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2019-12-31 |
Packaging
NDC 70857-002-10
100 TABLET, CHEWABLE in 1 BOTTLE (70857-002-10)
| Marketing Start Date | 2016-10-03 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 70857-002-10 [70857000210]
Traulevium TABLET, CHEWABLE
| Marketing Category | unapproved homeopathic |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2016-10-03 |
Drug Details
Active Ingredients
| Ingredient | Strength |
|---|---|
| ATROPA BELLADONNA | 4 [hp_X]/1 |
OpenFDA Data
| SPL SET ID: | 6123d145-4edf-4ecd-8b30-196e52273127 |
| Manufacturer | |
| UNII | |
| UPC Code |
NDC Crossover Matching brand name "Traulevium" or generic name "Belladonna, Arnica Montana Radix, Aconitum Napellus, Chamomilla, Symphytum Officinale, Calendula Officinalis, Hamamelis Virginiana, Millefolium, Hepar Sulphuris Calcareum, Mercurius Solubilis, Hypericum Perforatum, Bellis Perennis, Echinacea Angustifolia, Echinacea Purpurea."
| NDC | Brand Name | Generic Name |
|---|---|---|
| 70857-001 | Traulevium | Arnica Montana Radix, Calendula Officinalis, Hamamelis Virginiana, Echinacea, Echinacea Purpurea, Chamomilla, Symphytum Officinale, Bellis Perennis, Hypericum Perforatum, Millefolium, Aconitum Napellus, Belladonna, Mercurius Solubilis, Hepar Sulphuris Calcareum. |
| 70857-002 | Traulevium | Belladonna, Arnica Montana Radix, Aconitum Napellus, Chamomilla, Symphytum Officinale, Calendula Officinalis, Hamamelis Virginiana, Millefolium, Hepar Sulphuris Calcareum, Mercurius Solubilis, Hypericum Perforatum, Bellis Perennis, Echinacea Angustifolia, Echinacea Purpurea. |
| 70857-003 | TRAULEVIUM | Arnica Rad, Aconitum Nap, Chamomilla, Belladonna, Symphytum, Bellis, Calendula, Echinacea, Echinacea Purp, Hamamelis, Hypericum, Millefolium, Hepar Sulph Calc, Merc Solub. |
| 70857-004 | Traulevium | Arnica Montana, Calendula Officinalis, Hamamelis Virginiana, Echinacea, Echinacea Purpurea, Chamomilla, Symphytum Officinale, Bellis Perennis, Hypericum Perforatum, Millefolium, Aconitum Napellus, Belladonna, Mercurius Solubilis, Hepar Sulphuris Calcareum. |
Trademark Results [Traulevium]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
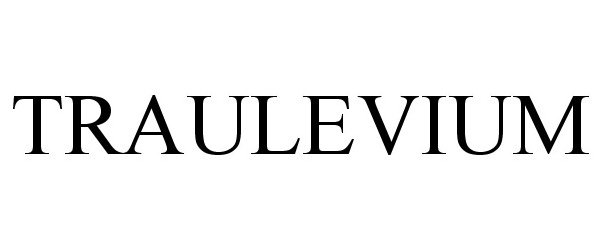 TRAULEVIUM 87171358 5186250 Live/Registered |
Medical Technology Products, Inc. 2016-09-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.