NDC 73006-101
Only For COUGH
Guaifenesin, Dextromethorphan Hbr
Only For COUGH is a Oral Tablet in the Human Otc Drug category. It is labeled and distributed by O4 Global Trading Usa, Llc. The primary component is Guaifenesin; Dextromethorphan Hydrobromide.
| Product ID | 73006-101_6c7abe44-03b7-4d12-a034-0bba3313a6ce |
| NDC | 73006-101 |
| Product Type | Human Otc Drug |
| Proprietary Name | Only For COUGH |
| Generic Name | Guaifenesin, Dextromethorphan Hbr |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2019-03-29 |
| Marketing Category | OTC MONOGRAPH FINAL / OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Labeler Name | O4 Global Trading Usa, Llc |
| Substance Name | GUAIFENESIN; DEXTROMETHORPHAN HYDROBROMIDE |
| Active Ingredient Strength | 400 mg/1; mg/1 |
| NDC Exclude Flag | N |
| Listing Certified Through | 2020-12-31 |
Packaging
NDC 73006-101-41
1 BLISTER PACK in 1 CARTON (73006-101-41) > 4 TABLET in 1 BLISTER PACK
| Marketing Start Date | 2019-03-29 |
| NDC Exclude Flag | N |
| Sample Package? | N |
NDC SPL Data Element Entries
NDC 73006-101-41 [73006010141]
Only For COUGH TABLET
| Marketing Category | OTC monograph final |
| Application Number | part341 |
| Product Type | HUMAN OTC DRUG |
| Marketing Start Date | 2019-03-29 |
Drug Details
NDC Crossover Matching brand name "Only For COUGH" or generic name "Guaifenesin, Dextromethorphan Hbr"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 73006-101 | Only For COUGH | Only For COUGH |
| 47682-217 | Medique Guaicon DMS | Guaifenesin, Dextromethorphan Hbr |
| 0904-6233 | MUCUS RELIEF DM | GUAIFENESIN, DEXTROMETHORPHAN HBr |
| 11822-0733 | Mucus Relief DM | Guaifenesin, Dextromethorphan HBr |
| 11822-7340 | Mucus Relief DM | Guaifenesin, Dextromethorphan HBr |
| 21130-933 | Mucus Relief DM | Guaifenesin, Dextromethorphan HBr |
| 37012-729 | Mucus Relief DM | Guaifenesin, Dextromethorphan HBr |
| 49035-825 | Mucus Relief DM | Guaifenesin, Dextromethorphan HBr |
| 11673-833 | Mucus Relief DM Extended Release | Guaifenesin, Dextromethorphan HBr |
| 0363-0437 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 0363-0733 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 0536-1161 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 0536-1213 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 37012-730 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 41250-633 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 41250-934 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 49035-824 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
| 50090-6020 | Mucus Relief DM Extended Release Caplets | Guaifenesin, Dextromethorphan HBr |
Trademark Results [Only For]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
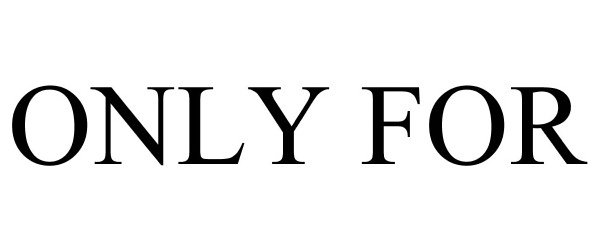 ONLY FOR 88231810 not registered Live/Pending |
Only For Licensing México, S.A.P.I. de C.V. 2018-12-17 |
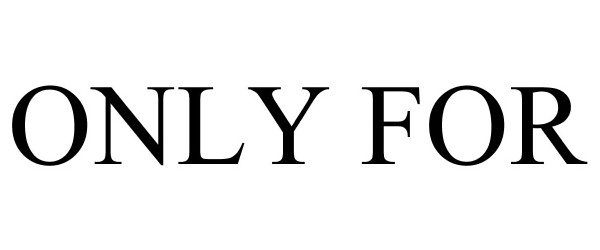 ONLY FOR 86706651 5745769 Live/Registered |
ONLY FOR LICENSING MEXICO, S.A.P.I DE C.V. 2015-07-28 |
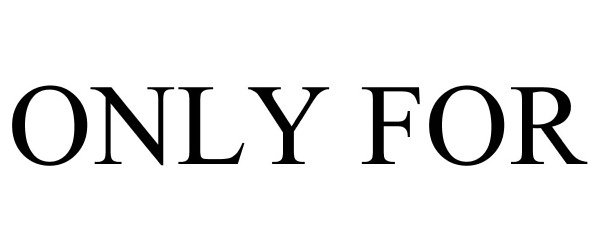 ONLY FOR 86689761 not registered Live/Pending |
BOUG-MEX CORP 2015-07-10 |
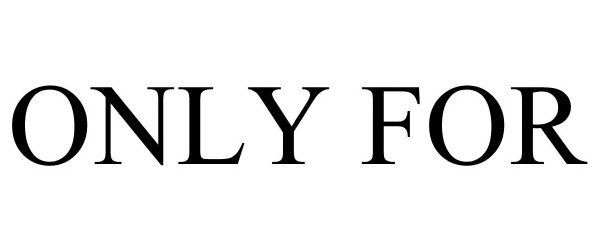 ONLY FOR 85196301 not registered Dead/Abandoned |
GMSC, LLC 2010-12-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.