NDC 76348-540
Hemporrhoid
Lidocaine Hcl
Hemporrhoid is a Topical Cream in the Human Otc Drug category. It is labeled and distributed by Renu Laboratories, Inc.. The primary component is Lidocaine Hydrochloride.
| Product ID | 76348-540_ab6f128a-a5a6-7ba8-e053-2a95a90a87d8 |
| NDC | 76348-540 |
| Product Type | Human Otc Drug |
| Proprietary Name | Hemporrhoid |
| Generic Name | Lidocaine Hcl |
| Dosage Form | Cream |
| Route of Administration | TOPICAL |
| Marketing Start Date | 2020-07-28 |
| Marketing Category | OTC MONOGRAPH FINAL / |
| Application Number | part346 |
| Labeler Name | Renu Laboratories, Inc. |
| Substance Name | LIDOCAINE HYDROCHLORIDE |
| Active Ingredient Strength | 1 g/28g |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Drug Details
NDC Crossover Matching brand name "Hemporrhoid" or generic name "Lidocaine Hcl"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 76348-590 | Hemporrhoid | Hemporrhoid |
| 41250-093 | Aloe Vera Gel | Lidocaine HCl |
| 41167-0568 | Aspercreme Lidocaine No-Mess plus Lavender | Lidocaine HCl |
| 24909-701 | CBD Clinic - Mild Pain Therapy | Lidocaine HCL |
| 24909-761 | CBD Clinic Pain Relief - Level 1 | Lidocaine HCL |
| 24909-721 | CBD Medic - Odor-Free Serious Relief | Lidocaine HCL |
| 0363-8897 | Cooling Gel | Lidocaine HCl |
| 51350-012 | CURACAINE | LIDOCAINE HCL |
| 51316-022 | CVS Health Sunburn Relief Cooling Lidocaine 0.5 Pain-Relieving | Lidocaine HCl |
| 46122-614 | GNP Lidocaine | Lidocaine HCl |
| 50804-955 | Good Sense Burn Relief Aloe | Lidocaine HCl |
| 47682-320 | Green Guard Burn | Lidocaine HCl |
| 47682-330 | Green Guard Burn | Lidocaine HCl |
| 51662-1438 | LIDOCAINE HCl | LIDOCAINE HCl |
| 51662-1538 | LIDOCAINE HCl | LIDOCAINE HCl |
| 44577-704 | LidoStat | LIDOCAINE HCL |
| 47682-250 | Medi-First Burn | Lidocaine HCl |
| 47682-255 | Medi-First Burn | Lidocaine HCl |
| 10742-8386 | Mentholatum Lidocaine Heat | Lidocaine HCl |
| 36800-740 | Pain Relief | Lidocaine HCl |
| 11822-1116 | Pain Relief Roll-on | Lidocaine HCl |
| 50804-774 | Pain Relief Roll-on | Lidocaine HCl |
| 15127-002 | Select Brand Burn Relief | LIDOCAINE HCL |
| 0869-0005 | Sunburn Relief Gel | Lidocaine HCl |
| 0404-9974 | Xylocaine | Lidocaine HCl |
Trademark Results [Hemporrhoid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEMPORRHOID 88516253 not registered Live/Pending |
BBG Surgical LLC 2019-07-16 |
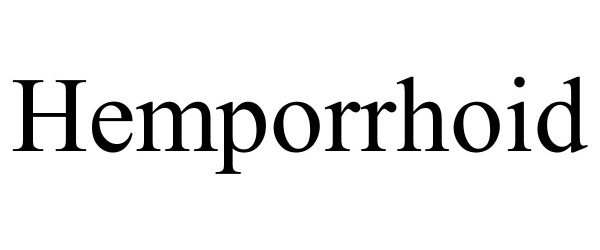 HEMPORRHOID 86610137 not registered Dead/Abandoned |
Haddad, Mark 2015-04-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.