NDC 78206-152
PROPECIA
Finasteride
PROPECIA is a Oral Tablet, Film Coated in the Human Prescription Drug category. It is labeled and distributed by Organon Llc. The primary component is Finasteride.
| Product ID | 78206-152_2b0a9a40-71a2-4df2-9b97-d86095e40863 |
| NDC | 78206-152 |
| Product Type | Human Prescription Drug |
| Proprietary Name | PROPECIA |
| Generic Name | Finasteride |
| Dosage Form | Tablet, Film Coated |
| Route of Administration | ORAL |
| Marketing Start Date | 2021-06-01 |
| Marketing Category | NDA / |
| Application Number | NDA020788 |
| Labeler Name | Organon LLC |
| Substance Name | FINASTERIDE |
| Active Ingredient Strength | 1 mg/1 |
| Pharm Classes | 5-alpha Reductase Inhibitor [EPC],5-alpha Reductase Inhibitors [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 78206-152-01
30 TABLET, FILM COATED in 1 BOTTLE (78206-152-01)
| Marketing Start Date | 2021-06-01 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "PROPECIA" or generic name "Finasteride"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0006-0071 | PROPECIA | finasteride |
| 0093-7355 | Finasteride | Finasteride |
| 0179-0175 | Finasteride | Finasteride |
| 0378-5036 | Finasteride | finasteride |
| 0904-6830 | Finasteride | Finasteride |
| 16729-089 | Finasteride | Finasteride |
| 16729-090 | Finasteride | finasteride |
| 17856-0090 | Finasteride | finasteride |
| 31722-525 | Finasteride | Finasteride |
| 31722-526 | Finasteride | Finasteride |
| 33261-833 | Finasteride | Finasteride |
| 35573-400 | Finasteride | Finasteride |
| 42291-280 | Finasteride | Finasteride |
| 43598-303 | Finasteride | Finasteride |
| 43598-390 | Finasteride | Finasteride |
| 45963-500 | Finasteride | Finasteride |
| 45963-600 | FINASTERIDE | FINASTERIDE |
| 47335-714 | Finasteride | Finasteride |
| 47335-715 | FINASTERIDE | FINASTERIDE |
| 50090-1718 | Finasteride | finasteride |
| 0006-0072 | PROSCAR | FINASTERIDE |
Trademark Results [PROPECIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
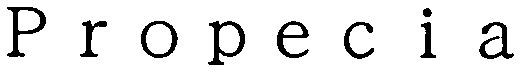 PROPECIA 79066804 not registered Dead/Abandoned |
ONODA Soushi 2009-02-23 |
 PROPECIA 74660644 2122033 Live/Registered |
MERCK SHARP & DOHME CORP. 1995-04-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.