NDC 80425-0103
Cleocin HCl
Clindamycin Hcl 300mg
Cleocin HCl is a Oral Capsule in the Human Prescription Drug category. It is labeled and distributed by Advanced Rx Pharmacy Of Tennessee, Llc. The primary component is Clindamycin Hydrochloride.
| Product ID | 80425-0103_b4f938b5-d981-449c-e053-2a95a90ae787 |
| NDC | 80425-0103 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Cleocin HCl |
| Generic Name | Clindamycin Hcl 300mg |
| Dosage Form | Capsule |
| Route of Administration | ORAL |
| Marketing Start Date | 2001-03-23 |
| Marketing Category | ANDA / ANDA |
| Application Number | ANDA065061 |
| Labeler Name | Advanced Rx Pharmacy of Tennessee, LLC |
| Substance Name | CLINDAMYCIN HYDROCHLORIDE |
| Active Ingredient Strength | 300 mg/1 |
| Pharm Classes | Decreased Sebaceous Gland Activity [PE],Lincosamide Antibacterial [EPC],Lincosamides [CS] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2021-12-31 |
Packaging
NDC 80425-0103-1
30 CAPSULE in 1 BOTTLE (80425-0103-1)
| Marketing Start Date | 2001-03-23 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
Trademark Results [Cleocin HCl]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
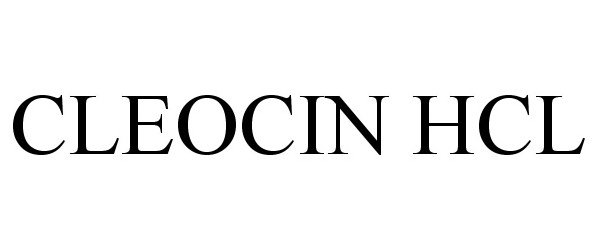 CLEOCIN HCL 86708327 not registered Dead/Abandoned |
Pharmacia & Upjohn Company LLC 2015-07-29 |
 CLEOCIN HCL 73443643 1314253 Live/Registered |
Upjohn Manufacturing Company; The 1983-09-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.