NDC 80725-005
Valium
Diazepam
Valium is a Oral Tablet in the Human Prescription Drug category. It is labeled and distributed by Waylis Therapuetics Llc. The primary component is Diazepam.
| Product ID | 80725-005_dc250376-39ef-2207-e053-2a95a90a4901 |
| NDC | 80725-005 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Valium |
| Generic Name | Diazepam |
| Dosage Form | Tablet |
| Route of Administration | ORAL |
| Marketing Start Date | 2022-04-04 |
| Marketing Category | NDA / |
| Application Number | NDA013263 |
| Labeler Name | Waylis Therapuetics LLC |
| Substance Name | DIAZEPAM |
| Active Ingredient Strength | 5 mg/1 |
| Pharm Classes | Benzodiazepine [EPC], Benzodiazepines [CS] |
| DEA Schedule | CIV |
| NDC Exclude Flag | N |
| Listing Certified Through | 2023-12-31 |
Packaging
NDC 80725-005-01
100 TABLET in 1 BOTTLE (80725-005-01)
| Marketing Start Date | 2022-04-04 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Valium" or generic name "Diazepam"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0140-0004 | Valium | diazepam |
| 0140-0005 | Valium | diazepam |
| 0140-0006 | Valium | diazepam |
| 55289-117 | Valium | Valium |
| 65084-313 | Valium | Valium |
| 65084-312 | Valium | Valium |
| 80425-0120 | Valium | Valium |
| 0187-0658 | Diastat | diazepam |
| 0187-0659 | Diastat | diazepam |
| 0054-3188 | Diazepam | Diazepam |
| 0093-6137 | Diazepam | Diazepam |
| 0093-6138 | Diazepam | Diazepam |
| 0093-6139 | Diazepam | Diazepam |
| 0121-0905 | Diazepam | Diazepam |
| 0172-3925 | Diazepam | Diazepam |
| 0172-3926 | Diazepam | Diazepam |
| 0172-3927 | Diazepam | Diazepam |
Trademark Results [Valium]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
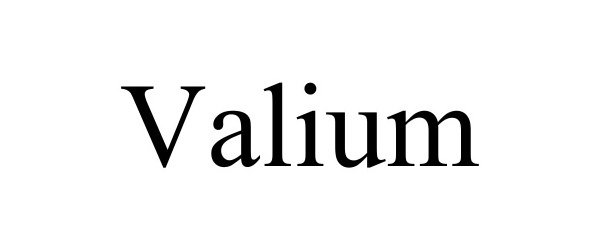 VALIUM 98704761 not registered Live/Pending |
Linder, Jacob 2024-08-19 |
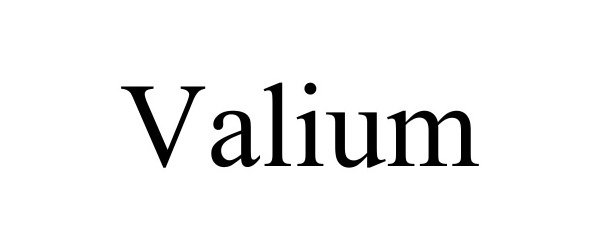 VALIUM 98644223 not registered Live/Pending |
Ghost Global LLC 2024-07-11 |
 VALIUM 72122442 0725548 Live/Registered |
HOFFMANN-LA ROCHE INC. 1961-06-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.