NDC 81749-004
Tarpeyo
Budesonide
Tarpeyo is a Oral Capsule, Delayed Release in the Human Prescription Drug category. It is labeled and distributed by Calliditas Therapeutics Ab. The primary component is Budesonide.
| Product ID | 81749-004_eba244a0-8fb9-4859-81b6-128213b80f35 |
| NDC | 81749-004 |
| Product Type | Human Prescription Drug |
| Proprietary Name | Tarpeyo |
| Generic Name | Budesonide |
| Dosage Form | Capsule, Delayed Release |
| Route of Administration | ORAL |
| Marketing Start Date | 2021-12-15 |
| Marketing Category | NDA / |
| Application Number | NDA215935 |
| Labeler Name | Calliditas Therapeutics AB |
| Substance Name | BUDESONIDE |
| Active Ingredient Strength | 4 mg/1 |
| Pharm Classes | Corticosteroid [EPC],Corticosteroid Hormone Receptor Agonists [MoA] |
| NDC Exclude Flag | N |
| Listing Certified Through | 2022-12-31 |
Packaging
NDC 81749-004-01
120 CAPSULE, DELAYED RELEASE in 1 BOTTLE (81749-004-01)
| Marketing Start Date | 2021-12-15 |
| NDC Exclude Flag | N |
| Sample Package? | N |
Drug Details
NDC Crossover Matching brand name "Tarpeyo" or generic name "Budesonide"
| NDC | Brand Name | Generic Name |
|---|---|---|
| 0093-6815 | Budesonide | Budesonide |
| 0093-6816 | Budesonide | Budesonide |
| 0093-6817 | Budesonide | Budesonide |
| 0093-7445 | Budesonide | Budesonide |
| 0115-1687 | Budesonide Inhalation | Budesonide |
| 0115-1689 | Budesonide Inhalation | Budesonide |
| 0186-0916 | PULMICORT | Budesonide |
| 0186-0917 | PULMICORT | Budesonide |
| 0186-1988 | PULMICORT RESPULES | Budesonide |
| 0186-1989 | PULMICORT RESPULES | Budesonide |
| 0186-1990 | PULMICORT RESPULES | Budesonide |
Trademark Results [Tarpeyo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
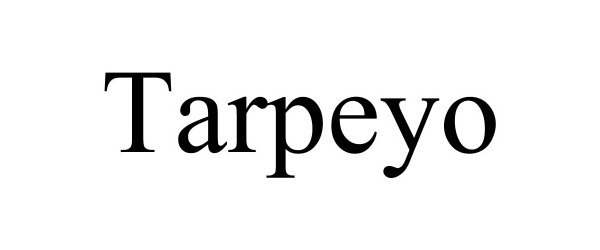 TARPEYO 88861008 not registered Live/Pending |
Calliditas Therapeutics AB 2020-04-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.