NEXT-GEN 44-100XLBK
GUDID 00850043811886
X-GEN Powder-Free Nitrile Examination Gloves, Size XL, Black, 100
Genesee Scientific Holdings, LLC
Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial Nitrile examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | 00850043811886 |
| NIH Device Record Key | 846003cd-4794-4764-a873-581160a2b7da |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | NEXT-GEN |
| Version Model Number | 44-100XLBK |
| Catalog Number | 44-100XLBK |
| Company DUNS | 943016071 |
| Company Name | Genesee Scientific Holdings, LLC |
| Device Count | 100 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00850043811879 [Primary] |
| GS1 | 00850043811886 [Package] Contains: 00850043811879 Package: [10 Units] In Commercial Distribution |
| GS1 | 10850043811876 [Unit of Use] |
FDA Product Code
| LZA | Polymer Patient Examination Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-04-24 |
| Device Publish Date | 2024-04-16 |
On-Brand Devices [NEXT-GEN]
| 00850043811503 | E-GEN Powder-Free Latex Examination Gloves, Size L, 100 |
| 10850043811494 | S-GEN Powder-Free Nitrile Examination Gloves, Size XS, 200 |
| 10850043811487 | S-GEN Powder-Free Nitrile Examination Gloves, Size XL, 200 |
| 10850043811470 | S-GEN Powder-Free Nitrile Examination Gloves, Size S, 200 |
| 10850043811463 | S-GEN Powder-Free Nitrile Examination Gloves, Size M, 200 |
| 10850043811456 | S-GEN Powder-Free Nitrile Examination Gloves, Size L, 200 |
| 10850043811449 | N-GEN Powder-Free Nitrile Examination Gloves, Size XS, 100 |
| 10850043811432 | N-GEN Powder-Free Nitrile Examination Gloves, Size XL, 100 |
| 10850043811425 | N-GEN Powder-Free Nitrile Examination Gloves, Size S, 100 |
| 10850043811418 | N-GEN Powder-Free Nitrile Examination Gloves, Size M, 100 |
| 10850043811401 | N-GEN Powder-Free Nitrile Examination Gloves, Size L, 100 |
| 10850043811395 | X-GEN Powder-Free Nitrile Examination Gloves, Size XS, 100 |
| 10850043811388 | X-GEN Powder-Free Nitrile Examination Gloves, Size XL, 100 |
| 10850043811371 | X-GEN Powder-Free Nitrile Examination Gloves, Size S, 100 |
| 10850043811364 | X-GEN Powder-Free Nitrile Examination Gloves, Size M, 100 |
| 10850043811357 | X-GEN Powder-Free Nitrile Examination Gloves, Size L, 100 |
| 10850043811340 | E-GEN Powder-Free Latex Examination Gloves, Size XS, 100 |
| 10850043811333 | E-GEN Powder-Free Latex Examination Gloves, Size XL, 100 |
| 10850043811326 | E-GEN Powder-Free Latex Examination Gloves, Size S, 100 |
| 10850043811319 | E-GEN Powder-Free Latex Examination Gloves, Size M, 100 |
| 10850043811296 | Z-GEN Powder-Free Nitrile Examination Gloves, Size L, 100 |
| 10850043811289 | Z-GEN Powder-Free Nitrile Examination Gloves, Size M, 100 |
| 10850043811272 | Z-GEN Powder-Free Nitrile Examination Gloves, Size S, 100 |
| 10850043811265 | Z-GEN Powder-Free Nitrile Examination Gloves, Size XL, 100 |
| 10850043811258 | Z-GEN Powder-Free Nitrile Examination Gloves, Size XS, 100 |
| 00850043811909 | X-GEN Powder-Free Nitrile Examination Gloves, Size XS, Black, 100 |
| 00850043811886 | X-GEN Powder-Free Nitrile Examination Gloves, Size XL, Black, 100 |
| 00850043811862 | X-GEN Powder-Free Nitrile Examination Gloves, Size S, Black, 100 |
| 00850043811848 | X-GEN Powder-Free Nitrile Examination Gloves, Size M, Black, 100 |
| 10850043811814 | X-GEN Powder-Free Nitrile Examination Gloves, Size L, Black, 100 |
Trademark Results [NEXT-GEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEXT-GEN 97274349 not registered Live/Pending |
Distributor Data Solutions, LLC 2022-02-18 |
 NEXT-GEN 87603488 5447810 Live/Registered |
Mota Group, Inc. 2017-09-11 |
 NEXT-GEN 85514806 not registered Dead/Abandoned |
Heinecke, Stuart 2012-01-12 |
 NEXT-GEN 77137106 3439167 Live/Registered |
Genesee Scientific Corporation 2007-03-21 |
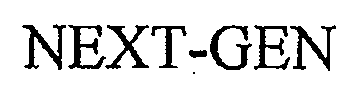 NEXT-GEN 76702555 not registered Dead/Abandoned |
UST GLOBAL Inc 2010-04-19 |
© 2024 FDA.report
This site is not affiliated with or endorsed by the FDA.