BACTOSHIELD CHG- chlorhexidine gluconate sponge
Bactoshield CHG by
Drug Labeling and Warnings
Bactoshield CHG by is a Otc medication manufactured, distributed, or labeled by Deb USA, Inc., Bajaj Medical , LLC, Biomed Packaging Systems, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Allergy alert
This product may cause a severe allergic realction. Symptoms may include:
- wheezing/difficulty breathing
- hives
- shock
- rash
- facial swelling
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- keep out of eyes, ears and mouth. May cause serious and permanent eye injury if permitted to enter or allowed to remain.
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- open package
- wet hands and forearms to the elbows with warm water
- clean under nails with nail pick provided. Nails should be maintained with a 1 millimeter free edge
- wet sponge and squeeze to work up lather
- scrub thoroughly for 3 minutes, paying particular attention to the nails, cuticles and interdigital spaces. Use the brush side to clean the nails, cuticles and interdigital spaces between the fingers and the sponge side to scrub the hands and forearms
- rinse thoroughly with warm water
- scrub for an additional 3 minutes using the sponge side
- discard brush-sponge
- rinse hands and forearms thoroughly and dry with a sterile towel
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL - 25 mL Applicator Box
Bactoshield® CHG 4%
deb med®
NDC: 11084-816-10
Chlorhexidine Gluconate 4% Solution
Surgical Hand ScrubSurgical Scrub
4% CHG
AntimicrobialREORDER #
1364-PJ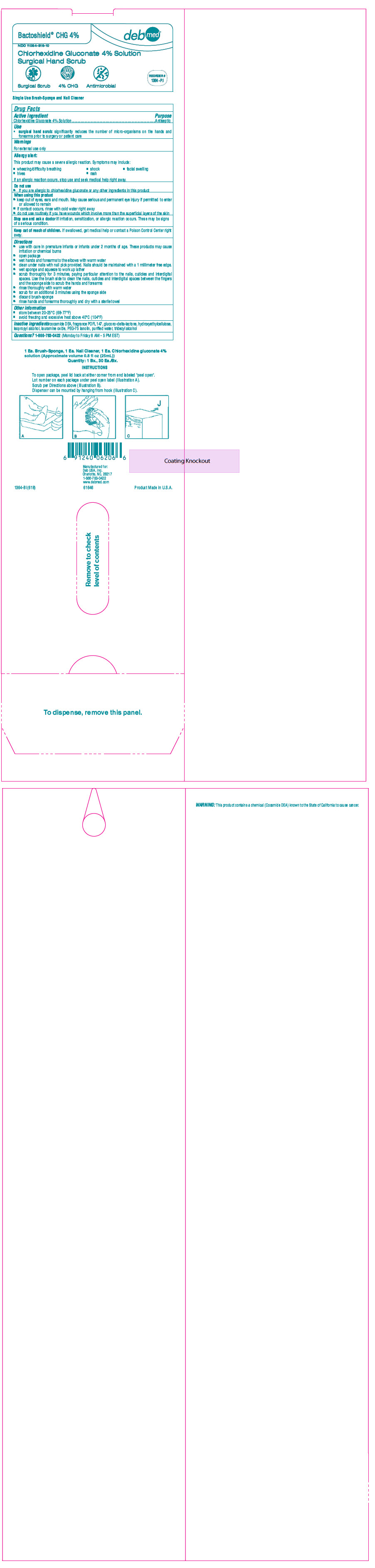
-
INGREDIENTS AND APPEARANCE
BACTOSHIELD CHG
chlorhexidine gluconate spongeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11084-816 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) GLUCONOLACTONE (UNII: WQ29KQ9POT) HYDROXYETHYL CELLULOSE (5000 MPA.S AT 1%) (UNII: X70SE62ZAR) ISOPROPYL ALCOHOL (UNII: ND2M416302) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) LANOLIN (UNII: 7EV65EAW6H) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (PALE) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11084-816-10 30 in 1 BOX 08/31/2018 1 NDC: 11084-816-09 25 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019822 08/31/2018 Labeler - Deb USA, Inc. (607378015) Establishment Name Address ID/FEI Business Operations Bajaj Medical , LLC 078774921 MANUFACTURE(11084-816) Establishment Name Address ID/FEI Business Operations Biomed Packaging Systems, Inc. 622463388 REPACK(11084-816) , RELABEL(11084-816)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.