PLAVIX- clopidogrel bisulfate tablet, film coated
Plavix by
Drug Labeling and Warnings
Plavix by is a Prescription medication manufactured, distributed, or labeled by Contract Pharmacy Services-PA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
PLAVIX safely and effectively. See full prescribing information for
PLAVIX.
PLAVIX (clopidogrel bisulfate) tablets
Initial U.S. Approval: 1997WARNING: DIMINISHED EFFECTIVENESS IN POOR METABOLIZERS
See full prescribing information for complete boxed warning.
- Effectiveness of Plavix depends on activation to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. (5.1)
- Poor metabolizers treated with Plavix at recommended doses exhibit higher cardiovascular event rates following acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI) than patients with normal CYP2C19 function. (12.5)
- Tests are available to identify a patient's CYP2C19 genotype and can be used as an aid in determining therapeutic strategy. (12.5)
- Consider alternative treatment or treatment strategies in patients identified as CYP2C19 poor metabolizers. (2.3, 5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Plavix is a P2Y12 platelet inhibitor indicated for:
- Acute coronary syndrome
- Recent myocardial infarction (MI), recent stroke, or established peripheral arterial disease. Plavix has been shown to reduce the combined endpoint of new ischemic stroke (fatal or not), new MI (fatal or not), and other vascular death. (1.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 75 mg, 300 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Reduced effectiveness in impaired CYP2C19 function: Avoid concomitant use with drugs that inhibit CYP2C19 (e.g., omeprazole). (5.1)
- Bleeding: Plavix increases risk of bleeding. Discontinue 5 days prior to elective surgery. (5.2)
- Discontinuation of Plavix: Premature discontinuation increases risk of cardiovascular events. (5.3)
- Recent transient ischemic attack or stroke: Combination use of Plavix and aspirin in these patients was not shown to be more effective than Plavix alone, but was shown to increase major bleeding. (5.4)
- Thrombotic thrombocytopenic purpura (TTP): TTP has been reported with Plavix, including fatal cases. (5.5)
ADVERSE REACTIONS
Bleeding, including life-threatening and fatal bleeding, is the most commonly reported adverse reaction. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Nursing mothers: Discontinue drug or nursing, taking into consideration importance of drug to mother. (8.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DIMINISHED EFFECTIVENESS IN POOR METABOLIZERS
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
1.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
2 DOSAGE AND ADMINISTRATION
2.1 Acute Coronary Syndrome
2.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
2.3 CYP2C19 Poor Metabolizers
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Active Bleeding
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Diminished Antiplatelet Activity Due to Impaired CYP2C19 Function
5.2 General Risk of Bleeding
5.3 Discontinuation of Plavix
5.4 Patients with Recent Transient Ischemic Attack (TIA) or Stroke
5.5 Thrombotic Thrombocytopenic Purpura (TTP)
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP2C19 Inhibitors
7.2 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
7.3 Warfarin (CYP2C9 Substrates)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Acute Coronary Syndrome
14.2 Recent Myocardial Infarction, Recent Stroke, or Established Peripheral Arterial Disease
14.3 Lack of Established Benefit of Plavix plus Aspirin in Patients with Multiple Risk Factors or Established Vascular Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Benefits and Risks
17.2 Bleeding
17.3 Other Signs and Symptoms Requiring Medical Attention
17.4 Invasive Procedures
17.5 Concomitant Medications
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DIMINISHED EFFECTIVENESS IN POOR METABOLIZERS
The effectiveness of Plavix is dependent on its activation to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19 [see Warnings and Precautions (5.1)]. Plavix at recommended doses forms less of that metabolite and has a smaller effect on platelet function in patients who are CYP2C19 poor metabolizers. Poor metabolizers with acute coronary syndrome or undergoing percutaneous coronary intervention treated with Plavix at recommended doses exhibit higher cardiovascular event rates than do patients with normal CYP2C19 function. Tests are available to identify a patient's CYP2C19 genotype; these tests can be used as an aid in determining therapeutic strategy [see Clinical Pharmacology (12.5)]. Consider alternative treatment or treatment strategies in patients identified as CYP2C19 poor metabolizers [see Dosage and Administration (2.3)].
-
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
- For patients with non-ST-segment elevation ACS [unstable angina (UA)/non-ST-elevation myocardial infarction (NSTEMI)], including patients who are to be managed medically and those who are to be managed with coronary revascularization, Plavix has been shown to decrease the rate of a combined endpoint of cardiovascular death, myocardial infarction (MI), or stroke as well as the rate of a combined endpoint of cardiovascular death, MI, stroke, or refractory ischemia.
- For patients with ST-elevation myocardial infarction (STEMI), Plavix has been shown to reduce the rate of death from any cause and the rate of a combined endpoint of death, re-infarction, or stroke. The benefit for patients who undergo primary percutaneous coronary intervention is unknown.
The optimal duration of Plavix therapy in ACS is unknown.
1.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
For patients with a history of recent myocardial infarction (MI), recent stroke, or established peripheral arterial disease, Plavix has been shown to reduce the rate of a combined endpoint of new ischemic stroke (fatal or not), new MI (fatal or not), and other vascular death.
-
2 DOSAGE AND ADMINISTRATION
2.1 Acute Coronary Syndrome
Plavix can be administered with or without food [see Clinical Pharmacology (12.3)]
- For patients with non-ST-elevation ACS (UA/NSTEMI), initiate Plavix with a single 300 mg oral loading dose and then continue at 75 mg once daily. Initiate aspirin (75–325 mg once daily) and continue in combination with Plavix [see Clinical Studies (14.1)].
- For patients with STEMI, the recommended dose of Plavix is 75 mg once daily orally, administered in combination with aspirin (75–325 mg once daily), with or without thrombolytics. Plavix may be initiated with or without a loading dose [see Clinical Studies (14.1)].
2.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
The recommended daily dose of Plavix is 75 mg once daily orally, with or without food [see Clinical Pharmacology (12.3)].
2.3 CYP2C19 Poor Metabolizers
CYP2C19 poor metabolizer status is associated with diminished antiplatelet response to clopidogrel. Although a higher dose regimen (600 mg loading dose followed by 150 mg once daily) in poor metabolizers increases antiplatelet response [see Clinical Pharmacology (12.5)], an appropriate dose regimen for this patient population has not been established in clinical outcome trials.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Active Bleeding
Plavix is contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage.
4.2 Hypersensitivity
Plavix is contraindicated in patients with hypersensitivity (e.g., anaphylaxis) to clopidogrel or any component of the product [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Diminished Antiplatelet Activity Due to Impaired CYP2C19 Function
Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by genetic variations in CYP2C19 [see Boxed Warning] and by concomitant medications that interfere with CYP2C19. Avoid concomitant use of Plavix and drugs that inhibit CYP2C19 activity. Co-administration of Plavix with omeprazole, a proton pump inhibitor that is an inhibitor of CYP2C19, reduces the pharmacological activity of Plavix if given concomitantly or if given 12 hours apart [see Drug Interactions (7.1)].
5.2 General Risk of Bleeding
Thienopyridines, including Plavix, increase the risk of bleeding. If a patient is to undergo surgery and an antiplatelet effect is not desired, discontinue Plavix 5 days prior to surgery. In patients who stopped therapy more than five days prior to CABG the rates of major bleeding were similar (event rate 4.4% Plavix + aspirin; 5.3% placebo + aspirin). In patients who remained on therapy within five days of CABG, the major bleeding rate was 9.6% for Plavix + aspirin, and 6.3% for placebo + aspirin.
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7–10 days), so withholding a dose will not be useful in managing a bleeding event or the risk of bleeding associated with an invasive procedure. Because the half-life of clopidogrel's active metabolite is short, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 4 hours of the loading dose or 2 hours of the maintenance dose may be less effective.
5.3 Discontinuation of Plavix
Avoid lapses in therapy, and if Plavix must be temporarily discontinued, restart as soon as possible. Premature discontinuation of Plavix may increase the risk of cardiovascular events.
5.4 Patients with Recent Transient Ischemic Attack (TIA) or Stroke
In patients with recent TIA or stroke who are at high risk for recurrent ischemic events, the combination of aspirin and Plavix has not been shown to be more effective than Plavix alone, but the combination has been shown to increase major bleeding.
5.5 Thrombotic Thrombocytopenic Purpura (TTP)
TTP, sometimes fatal, has been reported following use of Plavix, sometimes after a short exposure (<2 weeks). TTP is a serious condition that requires urgent treatment including plasmapheresis (plasma exchange). It is characterized by thrombocytopenia, microangiopathic hemolytic anemia (schistocytes [fragmented RBCs] seen on peripheral smear), neurological findings, renal dysfunction, and fever [see Adverse Reactions (6.2)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Bleeding [see Warnings and Precautions (5.2)]
- Thrombotic thrombocytopenic purpura [see Warnings and Precautions (5.5)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Plavix has been evaluated for safety in more than 54,000 patients, including over 21,000 patients treated for 1 year or more. The clinically important adverse reactions observed in trials comparing Plavix plus aspirin to placebo plus aspirin and trials comparing Plavix alone to aspirin alone are discussed below.
Bleeding
CURE
In CURE, Plavix use with aspirin was associated with an increase in major bleeding (primarily gastrointestinal and at puncture sites) compared to placebo with aspirin (see Table 1). The incidence of intracranial hemorrhage (0.1%) and fatal bleeding (0.2%) were the same in both groups. Other bleeding events that were reported more frequently in the clopidogrel group were epistaxis, hematuria, and bruise.
The overall incidence of bleeding is described in Table 1.
Table 1: CURE Incidence of Bleeding Complications (% patients) Event Plavix
(+ aspirin)*Placebo
(+ aspirin)*p-value (n=6259) (n=6303) - * Other standard therapies were used as appropriate.
- † Life-threatening and other major bleeding.
- ‡ Major bleeding event rate for Plavix + aspirin was dose-dependent on aspirin: <100 mg = 2.6%; 100–200 mg = 3.5%; >200 mg = 4.9%
Major bleeding event rates for Plavix + aspirin by age were: <65 years = 2.5%, ≥65 to <75 years = 4.1%, ≥75 years = 5.9%- § Major bleeding event rate for placebo + aspirin was dose-dependent on aspirin: <100 mg = 2.0%; 100–200 mg = 2.3%; >200 mg = 4.0%
Major bleeding event rates for placebo + aspirin by age were: <65 years = 2.1%, ≥65 to <75 years = 3.1%, ≥75 years = 3.6%- ¶ Led to interruption of study medication.
Major bleeding † 3.7 ‡ 2.7 § 0.001 Life-threatening bleeding 2.2 1.8 0.13 Fatal 0.2 0.2 5 g/dL hemoglobin drop 0.9 0.9 Requiring surgical intervention 0.7 0.7 Hemorrhagic strokes 0.1 0.1 Requiring inotropes 0.5 0.5 Requiring transfusion (≥4 units) 1.2 1.0 Other major bleeding 1.6 1.0 0.005 Significantly disabling 0.4 0.3 Intraocular bleeding with significant loss of vision 0.05 0.03 Requiring 2–3 units of blood 1.3 0.9 Minor bleeding ¶ 5.1 2.4 < 0.001 Ninety-two percent (92%) of the patients in the CURE study received heparin or low molecular weight heparin (LMWH), and the rate of bleeding in these patients was similar to the overall results.
COMMIT
In COMMIT, similar rates of major bleeding were observed in the Plavix and placebo groups, both of which also received aspirin (see Table 2).
Table 2: Incidence of Bleeding Events in COMMIT (% patients) Type of bleeding Plavix
(+ aspirin)
(n=22961)Placebo
(+ aspirin)
(n=22891)p-value - * Major bleeds were cerebral bleeds or non-cerebral bleeds thought to have caused death or that required transfusion.
- † The relative rate of major noncerebral or cerebral bleeding was independent of age. Event rates for Plavix + aspirin by age were: <60 years = 0.3%, ≥60 to <70 years = 0.7%, ≥70 years = 0.8%. Event rates for placebo + aspirin by age were: <60 years = 0.4%, ≥60 to <70 years = 0.6%, ≥70 years = 0.7%.
Major* noncerebral or cerebral bleeding† 0.6 0.5 0.59 Major noncerebral 0.4 0.3 0.48 Fatal 0.2 0.2 0.90 Hemorrhagic stroke 0.2 0.2 0.91 Fatal 0.2 0.2 0.81 Other noncerebral bleeding (non-major) 3.6 3.1 0.005 Any noncerebral bleeding 3.9 3.4 0.004 CAPRIE (Plavix vs. Aspirin)
In CAPRIE, gastrointestinal hemorrhage occurred at a rate of 2.0% in those taking Plavix vs. 2.7% in those taking aspirin; bleeding requiring hospitalization occurred in 0.7% and 1.1%, respectively. The incidence of intracranial hemorrhage was 0.4% for Plavix compared to 0.5% for aspirin.
Other bleeding events that were reported more frequently in the Plavix group were epistaxis and hematoma.
Other Adverse Events
In CURE and CHARISMA, which compared Plavix plus aspirin to aspirin alone, there was no difference in the rate of adverse events (other than bleeding) between Plavix and placebo.
In CAPRIE, which compared Plavix to aspirin, pruritus was more frequently reported in those taking Plavix. No other difference in the rate of adverse events (other than bleeding) was reported.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Plavix. Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic system disorders: Agranulocytosis, aplastic anemia/pancytopenia, thrombotic thrombocytopenic purpura (TTP)
- Gastrointestinal disorders: Gastrointestinal and retroperitoneal hemorrhage with fatal outcome, colitis (including ulcerative or lymphocytic colitis), pancreatitis, stomatitis
- General disorders and administration site condition: Fever, hemorrhage of operative wound
- Hepato-biliary disorders: Acute liver failure, hepatitis (non-infectious), abnormal liver function test
- Immune system disorders: Hypersensitivity reactions, anaphylactoid reactions, serum sickness
- Musculoskeletal, connective tissue and bone disorders: Musculoskeletal bleeding, myalgia, arthralgia, arthritis
- Nervous system disorders: Taste disorders, fatal intracranial bleeding
- Eye disorders: Eye (conjunctival, ocular, retinal) bleeding
- Psychiatric disorders: Confusion, hallucinations
- Respiratory, thoracic and mediastinal disorders: Bronchospasm, interstitial pneumonitis, respiratory tract bleeding
- Renal and urinary disorders: Glomerulopathy, increased creatinine levels
- Skin and subcutaneous tissue disorders: Maculopapular or erythematous rash, urticaria, bullous dermatitis, eczema, toxic epidermal necrolysis, Stevens-Johnson syndrome, angioedema, erythema multiforme, skin bleeding, lichen planus
- Vascular disorders: Vasculitis, hypotension
-
7 DRUG INTERACTIONS
7.1 CYP2C19 Inhibitors
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of drugs that inhibit the activity of this enzyme results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition. Avoid concomitant use of drugs that inhibit CYP2C19, e.g., omeprazole [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.5)].
Omeprazole
In a crossover clinical study, 72 healthy subjects were administered Plavix (300 mg loading dose followed by 75 mg per day) alone and with omeprazole (80 mg at the same time as Plavix) for 5 days. The exposure to the active metabolite of clopidogrel was decreased by 46% (Day 1) and 42% (Day 5) when Plavix and omeprazole were administered together. Mean inhibition of platelet aggregation was diminished by 47% (24 hours) and 30% (Day 5) when Plavix and omeprazole were administered together.
In another study, 72 healthy subjects were given the same doses of Plavix and omeprazole but the drugs were administered 12 hours apart; the results were similar, indicating that administering Plavix and omeprazole at different times does not prevent their interaction [see Warnings and Precautions (5.1)].
7.2 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Coadministration of Plavix and NSAIDs increases the risk of gastrointestinal bleeding.
7.3 Warfarin (CYP2C9 Substrates)
Although the administration of clopidogrel 75 mg per day did not modify the pharmacokinetics of S-warfarin (a CYP2C9 substrate) or INR in patients receiving long-term warfarin therapy, coadministration of Plavix with warfarin increases the risk of bleeding because of independent effects on hemostasis.
However, at high concentrations in vitro, clopidogrel inhibits CYP2C9.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies performed in rats and rabbits at doses up to 500 and 300 mg/kg/day, respectively (65 and 78 times the recommended daily human dose, respectively, on a mg/m2 basis), revealed no evidence of impaired fertility or fetotoxicity due to clopidogrel. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of a human response, Plavix should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Studies in rats have shown that clopidogrel and/or its metabolites are excreted in the milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from clopidogrel, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
Of the total number of subjects in the CAPRIE and CURE controlled clinical studies, approximately 50% of patients treated with Plavix were 65 years of age and older, and 15% were 75 years and older. In COMMIT, approximately 58% of the patients treated with Plavix were 60 years and older, 26% of whom were 70 years and older.
The observed risk of thrombotic events with clopidogrel plus aspirin versus placebo plus aspirin by age category is provided in Figures 2 and 5 for the CURE and COMMIT trials, respectively [see Clinical Studies (14.1)]. The observed risk of bleeding events with clopidogrel plus aspirin versus placebo plus aspirin by age category is provided in Tables 1 and 2 for the CURE and COMMIT trials, respectively [see Adverse Reactions (6.1)]. No dosage adjustment is necessary in elderly patients.
8.6 Renal Impairment
Experience is limited in patients with severe and moderate renal impairment [see Clinical Pharmacology (12.2)].
8.7 Hepatic Impairment
No dosage adjustment is necessary in patients with hepatic impairment [see Clinical Pharmacology (12.2)].
-
10 OVERDOSAGE
Platelet inhibition by Plavix is irreversible and will last for the life of the platelet. Overdose following clopidogrel administration may result in bleeding complications. A single oral dose of clopidogrel at 1500 or 2000 mg/kg was lethal to mice and to rats and at 3000 mg/kg to baboons. Symptoms of acute toxicity were vomiting, prostration, difficult breathing, and gastrointestinal hemorrhage in animals.
Based on biological plausibility, platelet transfusion may restore clotting ability.
-
11 DESCRIPTION
Plavix (clopidogrel bisulfate) is a thienopyridine class inhibitor of P2Y12 ADP platelet receptors. Chemically it is methyl (+)-(S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C16H16ClNO2SH2SO4 and its molecular weight is 419.9.
The structural formula is as follows:
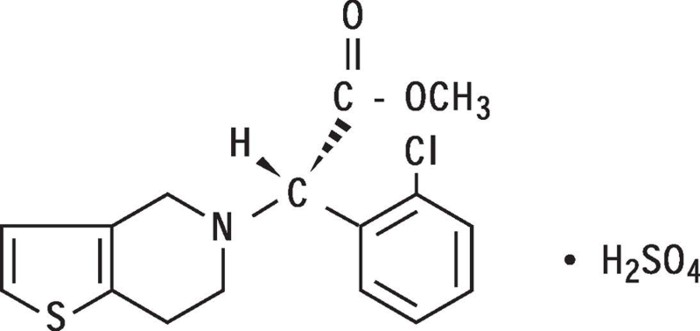
Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but freely soluble at pH 1. It also dissolves freely in methanol, dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether. It has a specific optical rotation of about +56°.
Plavix for oral administration is provided as either pink, round, biconvex, debossed, film-coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base or pink, oblong, debossed film-coated tablets containing 391.5 mg of clopidogrel bisulfate which is the molar equivalent of 300 mg of clopidogrel base.
Each tablet contains hydrogenated castor oil, hydroxypropylcellulose, mannitol, microcrystalline cellulose and polyethylene glycol 6000 as inactive ingredients. The pink film coating contains ferric oxide, hypromellose 2910, lactose monohydrate, titanium dioxide and triacetin. The tablets are polished with Carnauba wax.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clopidogrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
12.2 Pharmacodynamics
Clopidogrel must be metabolized by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation. The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. This action is irreversible. Consequently, platelets exposed to clopidogrel's active metabolite are affected for the remainder of their lifespan (about 7 to 10 days). Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation by released ADP.
Dose-dependent inhibition of platelet aggregation can be seen 2 hours after single oral doses of Plavix. Repeated doses of 75 mg Plavix per day inhibit ADP-induced platelet aggregation on the first day, and inhibition reaches steady state between Day 3 and Day 7. At steady state, the average inhibition level observed with a dose of 75 mg Plavix per day was between 40% and 60%. Platelet aggregation and bleeding time gradually return to baseline values after treatment is discontinued, generally in about 5 days.
Geriatric Patients
Elderly (≥75 years) and young healthy subjects had similar effects on platelet aggregation.
Renally-Impaired Patients
After repeated doses of 75 mg Plavix per day, patients with severe renal impairment (creatinine clearance from 5 to 15 mL/min) and moderate renal impairment (creatinine clearance from 30 to 60 mL/min) showed low (25%) inhibition of ADP-induced platelet aggregation.
Hepatically-Impaired Patients
After repeated doses of 75 mg Plavix per day for 10 days in patients with severe hepatic impairment, inhibition of ADP-induced platelet aggregation was similar to that observed in healthy subjects.
Gender
In a small study comparing men and women, less inhibition of ADP-induced platelet aggregation was observed in women.
12.3 Pharmacokinetics
Clopidogrel is a prodrug and is metabolized to a pharmacologically active metabolite and inactive metabolites.
Absorption
After single and repeated oral doses of 75 mg per day, clopidogrel is rapidly absorbed. Absorption is at least 50%, based on urinary excretion of clopidogrel metabolites.
Effect of Food
Plavix can be administered with or without food. In a study in healthy male subjects when Plavix 75 mg per day was given with a standard breakfast, mean inhibition of ADP-induced platelet aggregation was reduced by less than 9%. The active metabolite AUC0–24 was unchanged in the presence of food, while there was a 57% decrease in active metabolite Cmax. Similar results were observed when a Plavix 300 mg loading dose was administered with a high-fat breakfast.
Metabolism
Clopidogrel is extensively metabolized by two main metabolic pathways: one mediated by esterases and leading to hydrolysis into an inactive carboxylic acid derivative (85% of circulating metabolites) and one mediated by multiple cytochrome P450 enzymes. Cytochromes first oxidize clopidogrel to a 2-oxo-clopidogrel intermediate metabolite. Subsequent metabolism of the 2-oxo-clopidogrel intermediate metabolite results in formation of the active metabolite, a thiol derivative of clopidogrel. This metabolic pathway is mediated by CYP2C19, CYP3A, CYP2B6 and CYP1A2. The active thiol metabolite binds rapidly and irreversibly to platelet receptors, thus inhibiting platelet aggregation for the lifespan of the platelet.
The Cmax of the active metabolite is twice as high following a single 300 mg clopidogrel loading dose as it is after four days of 75 mg maintenance dose. Cmax occurs approximately 30 to 60 minutes after dosing. In the 75 to 300 mg dose range, the pharmacokinetics of the active metabolite deviates from dose proportionality: increasing the dose by a factor of four results in 2.0- and 2.7-fold increases in Cmax and AUC, respectively.
Elimination
Following an oral dose of 14C-labeled clopidogrel in humans, approximately 50% of total radioactivity was excreted in urine and approximately 46% in feces over the 5 days post-dosing. After a single, oral dose of 75 mg, clopidogrel has a half-life of approximately 6 hours. The half-life of the active metabolite is about 30 minutes.
12.5 Pharmacogenomics
CYP2C19 is involved in the formation of both the active metabolite and the 2-oxo-clopidogrel intermediate metabolite. Clopidogrel active metabolite pharmacokinetics and antiplatelet effects, as measured by ex vivo platelet aggregation assays, differ according to CYP2C19 genotype. Genetic variants of other CYP450 enzymes may also affect the formation of clopidogrel's active metabolite.
The CYP2C19*1 allele corresponds to fully functional metabolism while the CYP2C19*2 and *3 alleles are nonfunctional. CYP2C19*2 and *3 account for the majority of reduced function alleles in white (85%) and Asian (99%) poor metabolizers. Other alleles associated with absent or reduced metabolism are less frequent, and include, but are not limited to, CYP2C19*4, *5, *6, *7, and *8. A patient with poor metabolizer status will possess two loss-of-function alleles as defined above. Published frequencies for poor CYP2C19 metabolizer genotypes are approximately 2% for whites, 4% for blacks and 14% for Chinese. Tests are available to determine a patient's CYP2C19 genotype.
A crossover study in 40 healthy subjects, 10 each in the four CYP2C19 metabolizer groups, evaluated pharmacokinetic and antiplatelet responses using 300 mg followed by 75 mg per day and 600 mg followed by 150 mg per day, each for a total of 5 days. Decreased active metabolite exposure and diminished inhibition of platelet aggregation were observed in the poor metabolizers as compared to the other groups. When poor metabolizers received the 600 mg/150 mg regimen, active metabolite exposure and antiplatelet response were greater than with the 300 mg/75 mg regimen (see Table 3). An appropriate dose regimen for this patient population has not been established in clinical outcome trials.
Table 3: Active Metabolite Pharmacokinetics and Antiplatelet Responses by CYP2C19 Metabolizer Status Dose Ultrarapid
(n=10)Extensive
(n=10)Intermediate
(n=10)Poor
(n=10)Values are mean (SD) - * Inhibition of platelet aggregation with 5µM ADP; larger value indicates greater platelet inhibition
- † Vasodilator-stimulated phosphoprotein – platelet reactivity index; smaller value indicates greater platelet inhibition
Cmax (ng/mL) 300 mg (24 h) 24 (10) 32 (21) 23 (11) 11 (4) 600 mg (24 h) 36 (13) 44 (27) 39 (23) 17 (6) 75 mg (Day 5) 12 (6) 13 (7) 12 (5) 4 (1) 150 mg (Day 5) 16 (9) 19 (5) 18 (7) 7 (2) IPA (%)* 300 mg (24 h) 40 (21) 39 (28) 37 (21) 24 (26) 600 mg (24 h) 51 (28) 49 (23) 56 (22) 32 (25) 75 mg (Day 5) 56 (13) 58 (19) 60 (18) 37 (23) 150 mg (Day 5) 68 (18) 73 (9) 74 (14) 61 (14) VASP-PRI (%) † 300 mg (24 h) 73 (12) 68 (16) 77 (12) 91 (12) 600 mg (24 h) 51 (20) 48 (20) 56 (26) 85 (14) 75 mg (Day 5) 40 (9) 39 (14) 50 (16) 83 (13) 150 mg (Day 5) 20 (10) 24 (10) 29 (11) 61 (18) Some published studies suggest that intermediate metabolizers have decreased active metabolite exposure and diminished antiplatelet effects.
The relationship between CYP2C19 genotype and Plavix treatment outcome was evaluated in retrospective analyses of Plavix-treated subjects in CHARISMA (n=4862) and TRITON-TIMI 38 (n=1477), and in several published cohort studies. In TRITON-TIMI 38 and the majority of the cohort studies, the combined group of patients with either intermediate or poor metabolizer status had a higher rate of cardiovascular events (death, myocardial infarction, and stroke) or stent thrombosis compared to extensive metabolizers. In CHARISMA and one cohort study, the increased event rate was observed only in poor metabolizers.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to 77 mg/kg per day, which afforded plasma exposures >25 times that in humans at the recommended daily dose of 75 mg.
Clopidogrel was not genotoxic in four in vitro tests (Ames test, DNA-repair test in rat hepatocytes, gene mutation assay in Chinese hamster fibroblasts, and metaphase chromosome analysis of human lymphocytes) and in one in vivo test (micronucleus test by oral route in mice).
Clopidogrel was found to have no effect on fertility of male and female rats at oral doses up to 400 mg/kg per day (52 times the recommended human dose on a mg/m2 basis).
-
14 CLINICAL STUDIES
The clinical evidence of the efficacy of Plavix is derived from three double-blind trials involving 77,599 patients. The CAPRIE study (Clopidogrel vs. Aspirin in Patients at Risk of Ischemic Events) was a comparison of Plavix to aspirin. The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events) and the COMMIT/CCS-2 (Clopidogrel and Metoprolol in Myocardial Infarction Trial / Second Chinese Cardiac Study) studies were comparisons of Plavix to placebo, given in combination with aspirin and other standard therapy. The CHARISMA (Clopidogrel for High Atherothrombotic Risk Ischemic Stabilization, Management, and Avoidance) study (n=15,603) also compared Plavix to placebo, given in combination with aspirin and other standard therapy.
14.1 Acute Coronary Syndrome
CURE
The CURE study included 12,562 patients with ACS without ST-elevation (UA or NSTEMI) and presenting within 24 hours of onset of the most recent episode of chest pain or symptoms consistent with ischemia. Patients were required to have either ECG changes compatible with new ischemia (without ST-elevation) or elevated cardiac enzymes or troponin I or T to at least twice the upper limit of normal. The patient population was largely Caucasian (82%) and included 38% women, and 52% patients ≥65 years of age.
Patients were randomized to receive Plavix (300-mg loading dose followed by 75 mg once daily) or placebo, and were treated for up to one year. Patients also received aspirin (75–325 mg once daily) and other standard therapies such as heparin. The use of GPIIb/IIIa inhibitors was not permitted for three days prior to randomization.
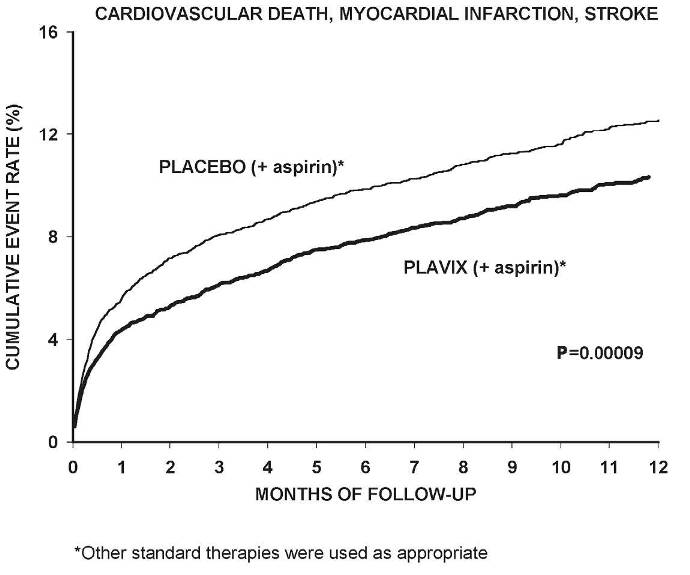
The number of patients experiencing the primary outcome (CV death, MI, or stroke) was 582 (9.3%) in the Plavix-treated group and 719 (11.4%) in the placebo-treated group, a 20% relative risk reduction (95% CI of 10%–28%; p < 0.001) for the Plavix-treated group (see Table 4).
Table 4: Outcome Events in the CURE Primary Analysis Outcome Plavix
(+ aspirin)*Placebo
(+ aspirin)*Relative Risk
Reduction (%)
(95% CI)(n=6259) (n=6303) - * Other standard therapies were used as appropriate.
- † The individual components do not represent a breakdown of the primary and co-primary outcomes, but rather the total number of subjects experiencing an event during the course of the study.
Primary outcome
(Cardiovascular death, MI, stroke)582 (9.3%) 719 (11.4%) 20%
(10.3, 27.9)
p < 0.001All Individual Outcome Events:† CV death 318 (5.1%) 345 (5.5%) 7%
(-7.7, 20.6)MI 324 (5.2%) 419 (6.6%) 23%
(11.0, 33.4)Stroke 75 (1.2%) 87 (1.4%) 14%
(-17.7, 36.6)Most of the benefit of Plavix occurred in the first two months, but the difference from placebo was maintained throughout the course of the trial (up to 12 months) (see Figure 1).
In CURE, the use of Plavix was associated with a lower incidence of CV death, MI or stroke in patient populations with different characteristics, as shown in Figure 2. The benefits associated with Plavix were independent of the use of other acute and long-term cardiovascular therapies, including heparin/LMWH, intravenous glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors, lipid-lowering drugs, beta-blockers, and ACE-inhibitors. The efficacy of Plavix was observed independently of the dose of aspirin (75–325 mg once daily). The use of oral anticoagulants, non-study anti-platelet drugs, and chronic NSAIDs was not allowed in CURE.
Figure 2: Hazard Ratio for Patient Baseline Characteristics and On-Study Concomitant Medications/Interventions for the CURE Study
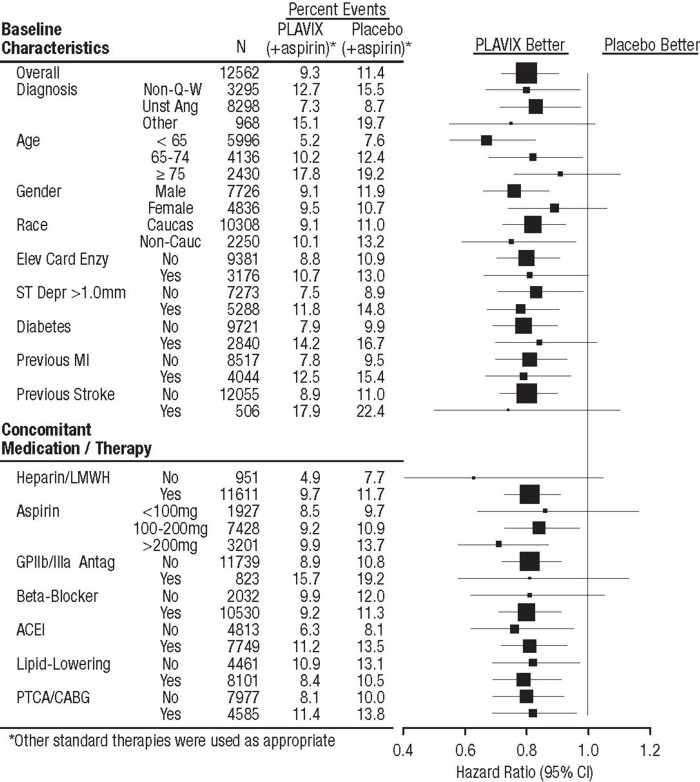
The use of Plavix in CURE was associated with a decrease in the use of thrombolytic therapy (71 patients [1.1%] in the Plavix group, 126 patients [2.0%] in the placebo group; relative risk reduction of 43%), and GPIIb/IIIa inhibitors (369 patients [5.9%] in the Plavix group, 454 patients [7.2%] in the placebo group, relative risk reduction of 18%). The use of Plavix in CURE did not affect the number of patients treated with CABG or PCI (with or without stenting), (2253 patients [36.0%] in the Plavix group, 2324 patients [36.9%] in the placebo group; relative risk reduction of 4.0%).
COMMIT
In patients with STEMI, the safety and efficacy of Plavix were evaluated in the randomized, placebo-controlled, double-blind study, COMMIT. COMMIT included 45,852 patients presenting within 24 hours of the onset of the symptoms of myocardial infarction with supporting ECG abnormalities (i.e., ST-elevation, ST-depression or left bundle-branch block). Patients were randomized to receive Plavix (75 mg once daily) or placebo, in combination with aspirin (162 mg per day), for 28 days or until hospital discharge, whichever came first.
The primary endpoints were death from any cause and the first occurrence of re-infarction, stroke or death.
The patient population included 28% women, 58% age ≥ 60 years (26% age ≥ 70 years), 55% patients who received thrombolytics, 68% who received ACE-inhibitors, and only 3% who underwent PCI.
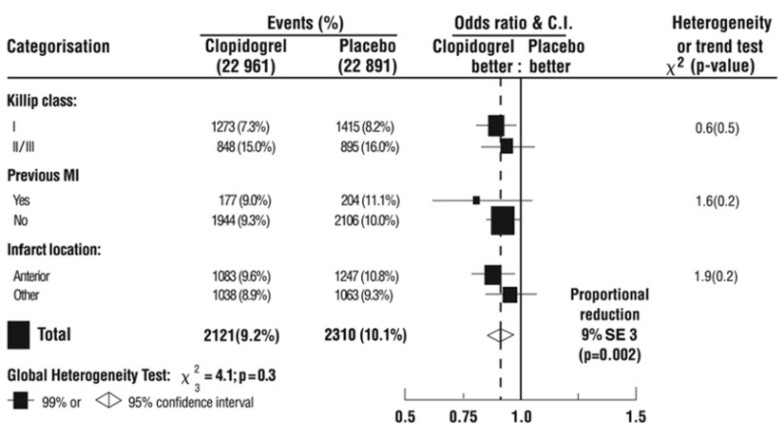
As shown in Table 5 and Figures 3 and 4 below, Plavix significantly reduced the relative risk of death from any cause by 7% (p=0.029), and the relative risk of the combination of re-infarction, stroke or death by 9% (p=0.002).
Table 5: Outcome Events in the COMMIT Analysis Event Plavix
(+ aspirin)
(N=22961)Placebo
(+ aspirin)
(N=22891)Odds ratio
(95% CI)p-value - * The difference between the composite endpoint and the sum of death+non-fatal MI+non-fatal stroke indicates that 9 patients (2 clopidogrel and 7 placebo) suffered both a non-fatal stroke and a non-fatal MI.
- † Non-fatal MI and non-fatal stroke exclude patients who died (of any cause).
Composite endpoint: Death, MI, or Stroke* 2121 (9.2%) 2310 (10.1%) 0.91 (0.86, 0.97) 0.002 Death 1726 (7.5%) 1845 (8.1%) 0.93 (0.87, 0.99) 0.029 Non-fatal MI† 270 (1.2%) 330 (1.4%) 0.81 (0.69, 0.95) 0.011 Non-fatal Stroke† 127 (0.6%) 142 (0.6%) 0.89 (0.70, 1.13) 0.33 Figure 3: Cumulative Event Rates for Death in the COMMIT Study 1
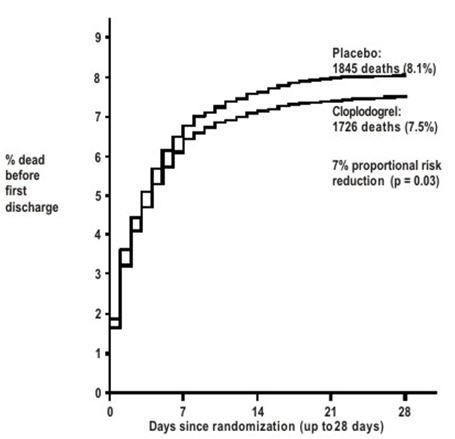
- 1 All treated patients received aspirin.
Figure 4: Cumulative Event Rates for the Combined End point Re-Infarction, Stroke or Death in the COMMIT Study 2
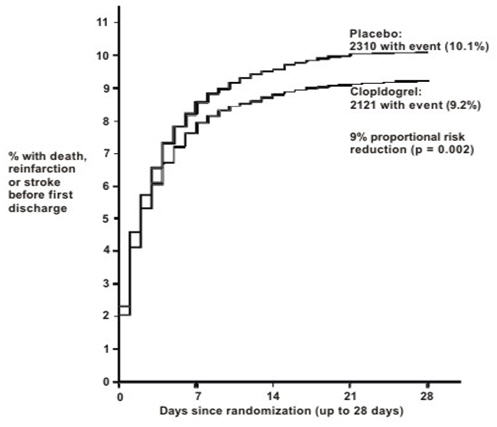
- 2 All treated patients received aspirin.
The effect of Plavix did not differ significantly in various pre-specified subgroups as shown in Figure 5. The effect was also similar in non-prespecified subgroups including those based on infarct location, Killip class or prior MI history (see Figure 6). Such subgroup analyses should be interpreted cautiously.
Figure 5: Effects of Adding Plavix to Aspirin on the Combined Primary Endpoint across Baseline and Concomitant Medication Subgroups for the COMMIT Study
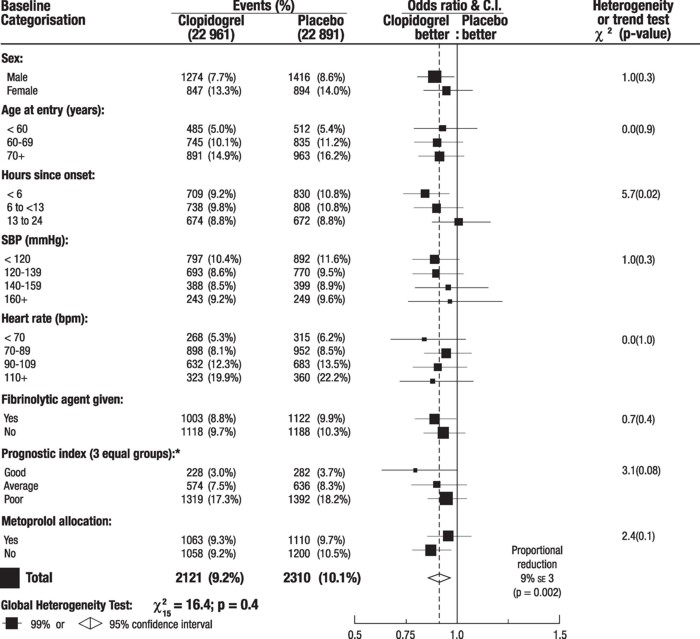
* Three similar-sized prognostic index groups were based on absolute risk of primary composite outcome for each patient calculated from baseline prognostic variables (excluding allocated treatments) with a Cox regression model.
14.2 Recent Myocardial Infarction, Recent Stroke, or Established Peripheral Arterial Disease
CAPRIE
The CAPRIE trial was a 19,185-patient, 304-center, international, randomized, double-blind, parallel-group study comparing Plavix (75 mg daily) to aspirin (325 mg daily). The patients randomized had: 1) recent histories of myocardial infarction (within 35 days); 2) recent histories of ischemic stroke (within 6 months) with at least a week of residual neurological signs; or 3) established peripheral arterial disease. Patients received randomized treatment for an average of 1.6 years (maximum of 3 years).
The trial's primary outcome was the time to first occurrence of new ischemic stroke (fatal or not), new myocardial infarction (fatal or not), or other vascular death. Deaths not easily attributable to nonvascular causes were all classified as vascular.
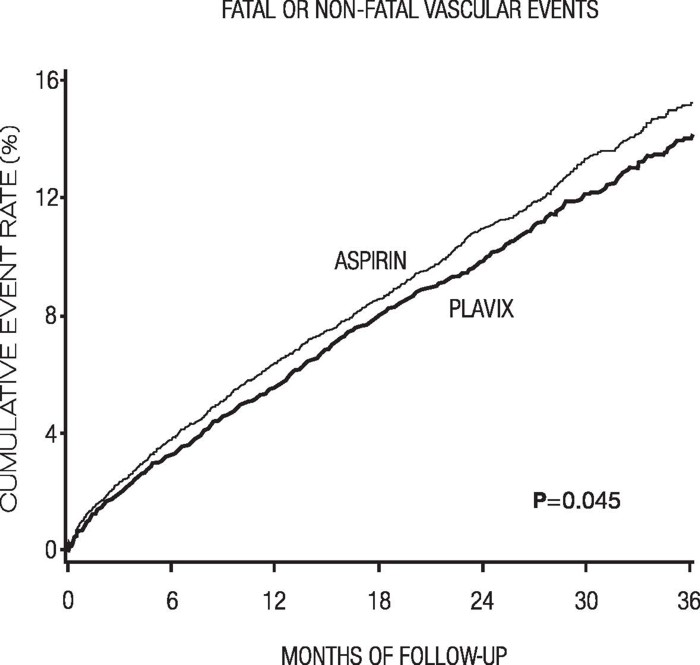
Table 6: Outcome Events in the CAPRIE Primary Analysis Plavix aspirin Patients n=9599 n=9586 Ischemic stroke (fatal or not) 438 (4.6%) 461 (4.8%) MI (fatal or not) 275 (2.9%) 333 (3.5%) Other vascular death 226 (2.4%) 226 (2.4%) Total 939 (9.8%) 1020 (10.6%) As shown in the table, Plavix was associated with a lower incidence of outcome events, primarily MI. The overall relative risk reduction (9.8% vs. 10.6%) was 8.7%, p=0.045. Similar results were obtained when all-cause mortality and all-cause strokes were counted instead of vascular mortality and ischemic strokes (risk reduction 6.9%). In patients who survived an on-study stroke or myocardial infarction, the incidence of subsequent events was lower in the Plavix group.
The curves showing the overall event rate are shown in Figure 7. The event curves separated early and continued to diverge over the 3-year follow-up period.
The statistical significance favoring Plavix over aspirin was marginal (p=0.045). However, because aspirin is itself effective in reducing cardiovascular events in patients with recent myocardial infarction or stroke, the effect of Plavix is substantial.
The CAPRIE trial included a population that was randomized on the basis of 3 entry criteria. The efficacy of Plavix relative to aspirin was heterogeneous across these randomized subgroups (p=0.043). It is not clear whether this difference is real or a chance occurrence. Although the CAPRIE trial was not designed to evaluate the relative benefit of Plavix over aspirin in the individual patient subgroups, the benefit appeared to be strongest in patients who were enrolled because of peripheral vascular disease (especially those who also had a history of myocardial infarction) and weaker in stroke patients. In patients who were enrolled in the trial on the sole basis of a recent myocardial infarction, Plavix was not numerically superior to aspirin.
14.3 Lack of Established Benefit of Plavix plus Aspirin in Patients with Multiple Risk Factors or Established Vascular Disease
CHARISMA
The CHARISMA trial was a 15,603 subject, randomized, double-blind, parallel group study comparing Plavix (75 mg daily) to placebo for prevention of ischemic events in patients with vascular disease or multiple risk factors for atherosclerosis. All subjects were treated with aspirin 75–162 mg daily. The mean duration of treatment was 23 months. The study failed to demonstrate a reduction in the occurrence of the primary endpoint, a composite of CV death, MI, or stroke. A total of 534 (6.9%) patients in the Plavix group versus 573 (7.4%) patients in the placebo group experienced a primary outcome event (p=0.22). Bleeding of all severities was more common in the subjects randomized to Plavix.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Plavix (clopidogrel bisulfate) 75 mg tablets are available as pink, round, biconvex, film-coated tablets debossed with "75" on one side and "1171" on the other. Tablets are provided as follows:
- NDC: 67046-604-30 Blisters of 30
-
17 PATIENT COUNSELING INFORMATION
17.1 Benefits and Risks
- Summarize the effectiveness features and potential side effects of Plavix.
- Tell patients to take Plavix exactly as prescribed.
- Remind patients not to discontinue Plavix without first discussing it with the physician who prescribed Plavix.
17.2 Bleeding
Inform patients that they:
- will bruise and bleed more easily.
- will take longer than usual to stop bleeding.
- should report any unanticipated, prolonged, or excessive bleeding, or blood in their stool or urine.
17.3 Other Signs and Symptoms Requiring Medical Attention
- Inform patients that TTP is a rare but serious condition that has been reported with Plavix and other drugs in this class of drugs.
- Instruct patients to get prompt medical attention if they experience any of the following symptoms that cannot otherwise be explained: fever, weakness, extreme skin paleness, purple skin patches, yellowing of the skin or eyes, or neurological changes.
17.4 Invasive Procedures
Instruct patients to:
- inform physicians and dentists that they are taking Plavix before any invasive procedure is scheduled.
- tell the doctor performing the invasive procedure to talk to the prescribing health care professional before stopping Plavix.
17.5 Concomitant Medications
Ask patients to list all prescription medications, over-the-counter medications, or dietary supplements they are taking or plan to take, including prescription or over-the-counter omeprazole, so the physician knows about other treatments that may affect how Plavix works (e.g., warfarin and NSAIDs) [see Warnings and Precautions (5)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PLAVIX
clopidogrel bisulfate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67046-604(NDC:63653-1171) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength clopidogrel bisulfate (UNII: 08I79HTP27) (clopidogrel - UNII:A74586SNO7) clopidogrel 75 mg Inactive Ingredients Ingredient Name Strength castor oil (UNII: D5340Y2I9G) hydroxypropyl cellulose (UNII: RFW2ET671P) mannitol (UNII: 3OWL53L36A) cellulose, microcrystalline (UNII: OP1R32D61U) polyethylene glycol 6000 (UNII: 30IQX730WE) ferric oxide red (UNII: 1K09F3G675) lactose monohydrate (UNII: EWQ57Q8I5X) titanium dioxide (UNII: 15FIX9V2JP) triacetin (UNII: XHX3C3X673) carnauba wax (UNII: R12CBM0EIZ) Product Characteristics Color PINK Score no score Shape ROUND (biconvex) Size 9mm Flavor Imprint Code 75;1171 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67046-604-30 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020839 02/26/1999 Labeler - Contract Pharmacy Services-PA (945429777)
Trademark Results [Plavix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PLAVIX 90168789 not registered Live/Pending |
Sanofi 2020-09-09 |
 PLAVIX 75443638 2320230 Live/Registered |
SANOFI 1998-03-03 |
 PLAVIX 75438881 2380297 Live/Registered |
SANOFI 1998-02-23 |
 PLAVIX 74482767 2042583 Live/Registered |
SANOFI 1994-01-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.