ASPIRIN ADULT LOW STRENGTH- aspirin tablet, delayed release
Aspirin by
Drug Labeling and Warnings
Aspirin by is a Otc medication manufactured, distributed, or labeled by Valu Merchandisers Company (Best Choice), P and L Development of New York Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
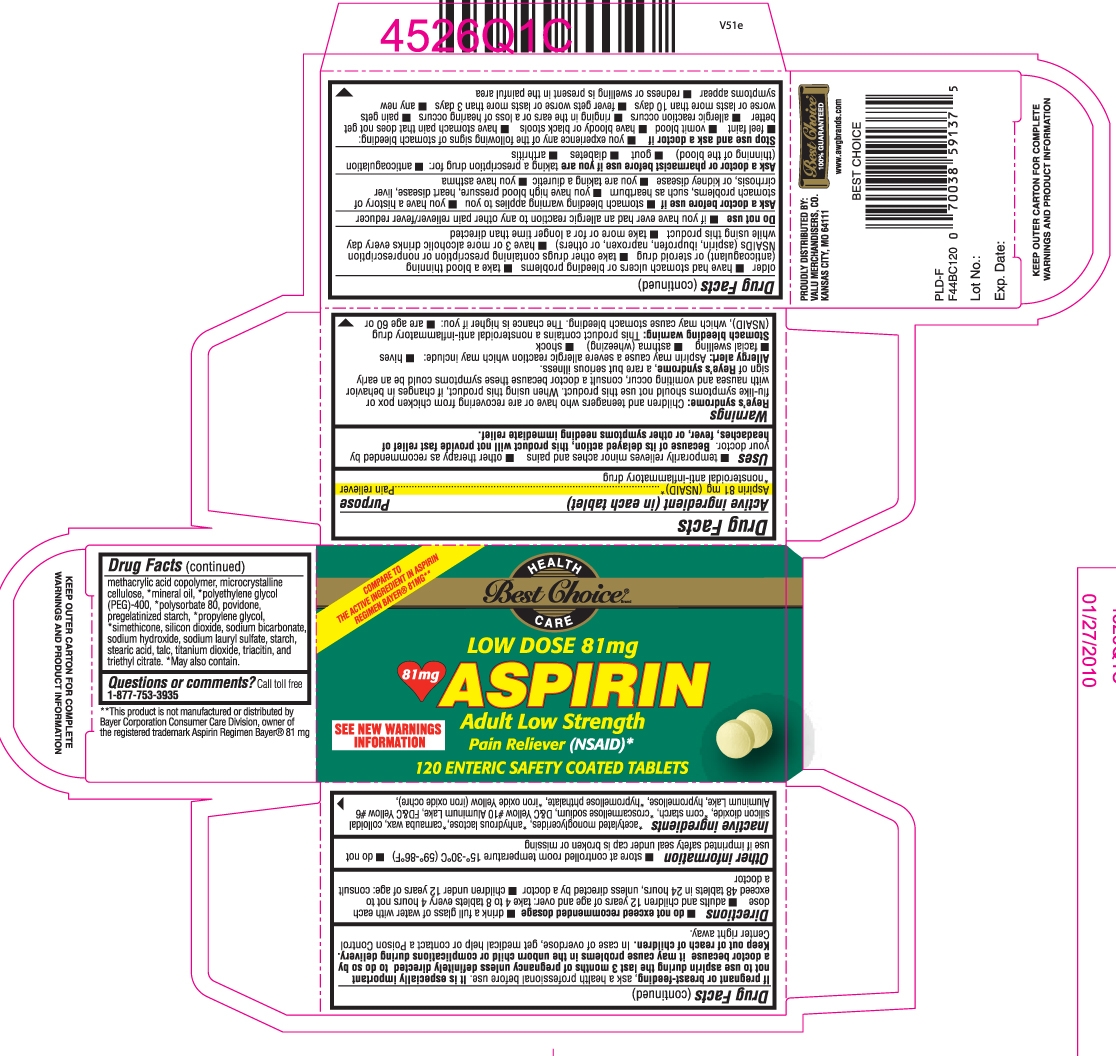
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not
use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these
symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma(wheezing)
- shock
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
-
you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
-
you are taking a diuretic
-
you have asthma
Ask a doctor or pharmacist before use if you are
taking a prescription drug for:
- anticoagulation (thinning of the blood)
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- allergic reaction occurs
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- any new symptoms appear
- redness or swelling is present in the painful area
- Directions
- Other information
-
Inactive ingredients
*acetylated monoglycerides, *anhydrous lactose, *carnauba wax, colloidal silicon dioxide,*corn starch, *croscarmellose sodium, D&C Yellow #10 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, hypromellose, *hypromellose phthalate, *iron oxide Yellow (iron
oxide ochre), methacrylic acid copolymer, microcrystalline cellulose, *mineral oil, *polyethylene glycol (PEG)-400, *polysorbate 80, povidone, pregelatinized starch, *propylene glycol, *simethicone, silicon dioxide, sodium bicarbonate, sodium hydroxide, sodium lauryl sulfate, starch, stearic acid, talc, titanium dioxide, triacetin, and triethyl citrate. *May also contain. - Questions or comments?
-
Principal Display Panel
Compare to the active ingredient in ASPIRIN REGIMEN BAYER® 81 mg**
SEE NEW WARNINGS INFORMATION
Low Dose 81 mg
ASPIRIN
adult low strength
Pain reliever (NSAID)*
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
**This product is not manufactured or distributedby Bayer Corporation Consumer care division, owner of the registered trademark Aspirin Regimen Bayer® 81 mg
- Product Label
-
INGREDIENTS AND APPEARANCE
ASPIRIN ADULT LOW STRENGTH
aspirin tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63941-440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength DIACETYLATED MONOGLYCERIDES (UNII: 5Z17386USF) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) HYPROMELLOSES (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONES (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIMETHICONE (UNII: 92RU3N3Y1O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color YELLOW Score no score Shape ROUND Size 10mm Flavor Imprint Code E;HEART;81 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63941-440-12 1 in 1 CARTON 1 120 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part343 07/26/2010 Labeler - Valu Merchandisers Company (Best Choice) (868703513) Registrant - P and L Development of New York Corporation (800014821)
Trademark Results [Aspirin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ASPIRIN 76481358 2788617 Dead/Cancelled |
Simon Carter Accessories, Ltd. 2003-01-10 |
 ASPIRIN 75209895 not registered Dead/Abandoned |
Bayer Aktiengesellschaft 1996-12-09 |
 ASPIRIN 73234351 1171777 Dead/Cancelled |
McIntyre; William A. 1979-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.