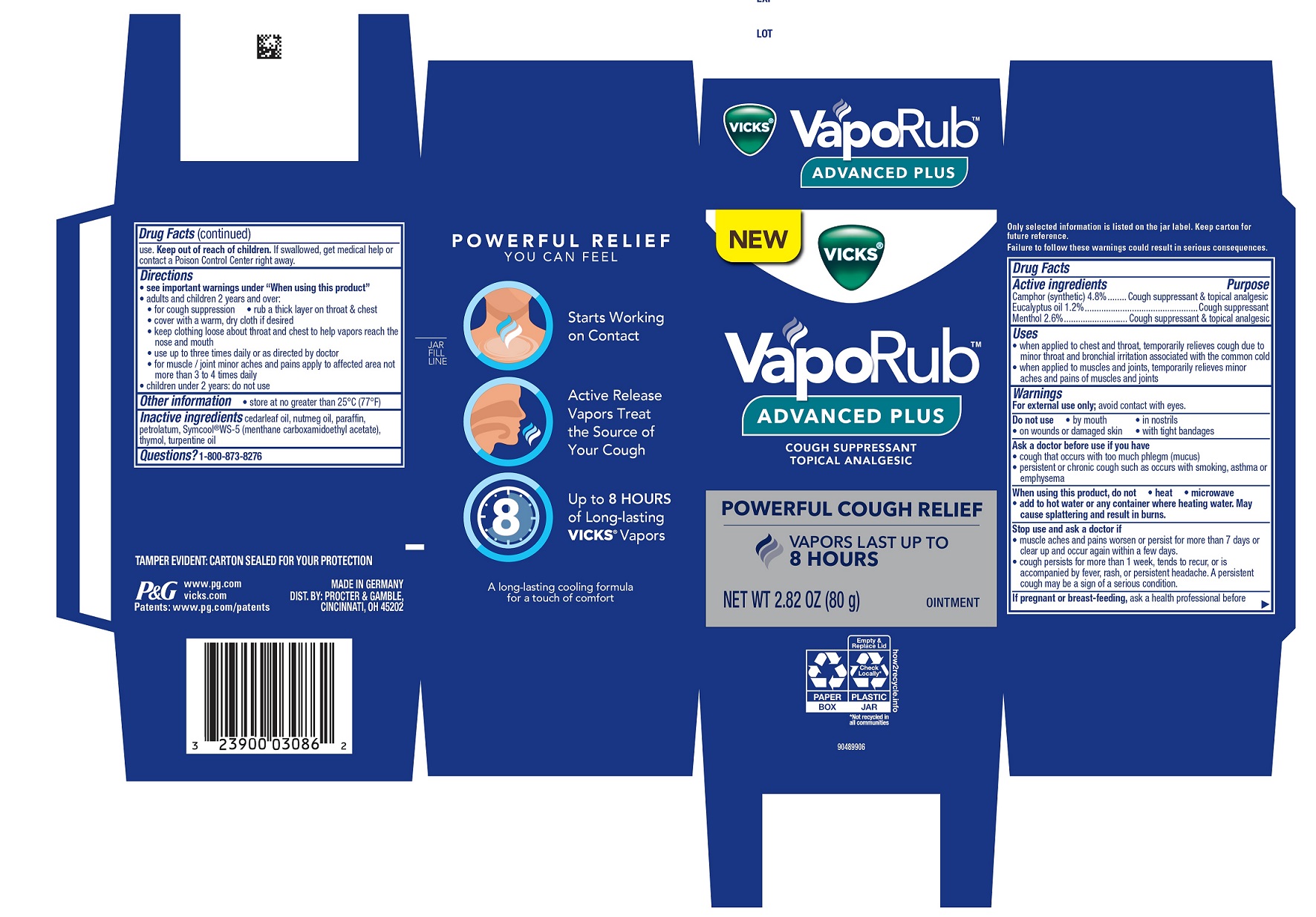
Vicks ®VapoRub™ ADVANCED PLUS
Vicks VapoRub ADVANCED PLUS by
Drug Labeling and Warnings
Vicks VapoRub ADVANCED PLUS by is a Otc medication manufactured, distributed, or labeled by Procter & Gamble Manufacturing Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VICKS VAPORUB ADVANCED PLUS- camphor, eucalyptus oil, menthol ointment
Procter & Gamble Manufacturing Company
----------
Vicks ®VapoRub™ ADVANCED PLUS
Active ingredients Purpose
Cough suppressant & topical analgesic
Cough suppressant
Cough suppressant & topical analgesic
Uses
when applied to chest and throat, temporarily relieves cough due to minor throat and bronchial irritation associated with the common cold
when applied to muscles and joints, temporarily relieves minor aches and pains of muscles and joints
Ask a doctor before use if you have
cough that occurs with too much phlegm (mucus)
persistent or chronic cough such as occurs with smoking, asthma or emphysema
When using this product
do not
heat
microwave
add to hot water or any container where heating water. May cause splattering and result in burns.
Stop use and ask a doctor if
muscle aches and pains worsen or persist for more than 7 days or clear up and occur again within a few days.
cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
Directions
see important warnings under “When using this product”
adults and children 2 years and over:
for cough suppression rub a thick layer on throat & chest
cover with a warm, dry cloth if desired
keep clothing loose about throat and chest to help vapors reach the nose and mouth
use up to three times daily or as directed by doctor
for muscle / joint minor aches and pains apply to affected area not more than 3 to 4 times daily
children under 2 years: do not use
| VICKS VAPORUB ADVANCED PLUS
camphor, eucalyptus oil, menthol ointment |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Procter & Gamble Manufacturing Company (004238200) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.