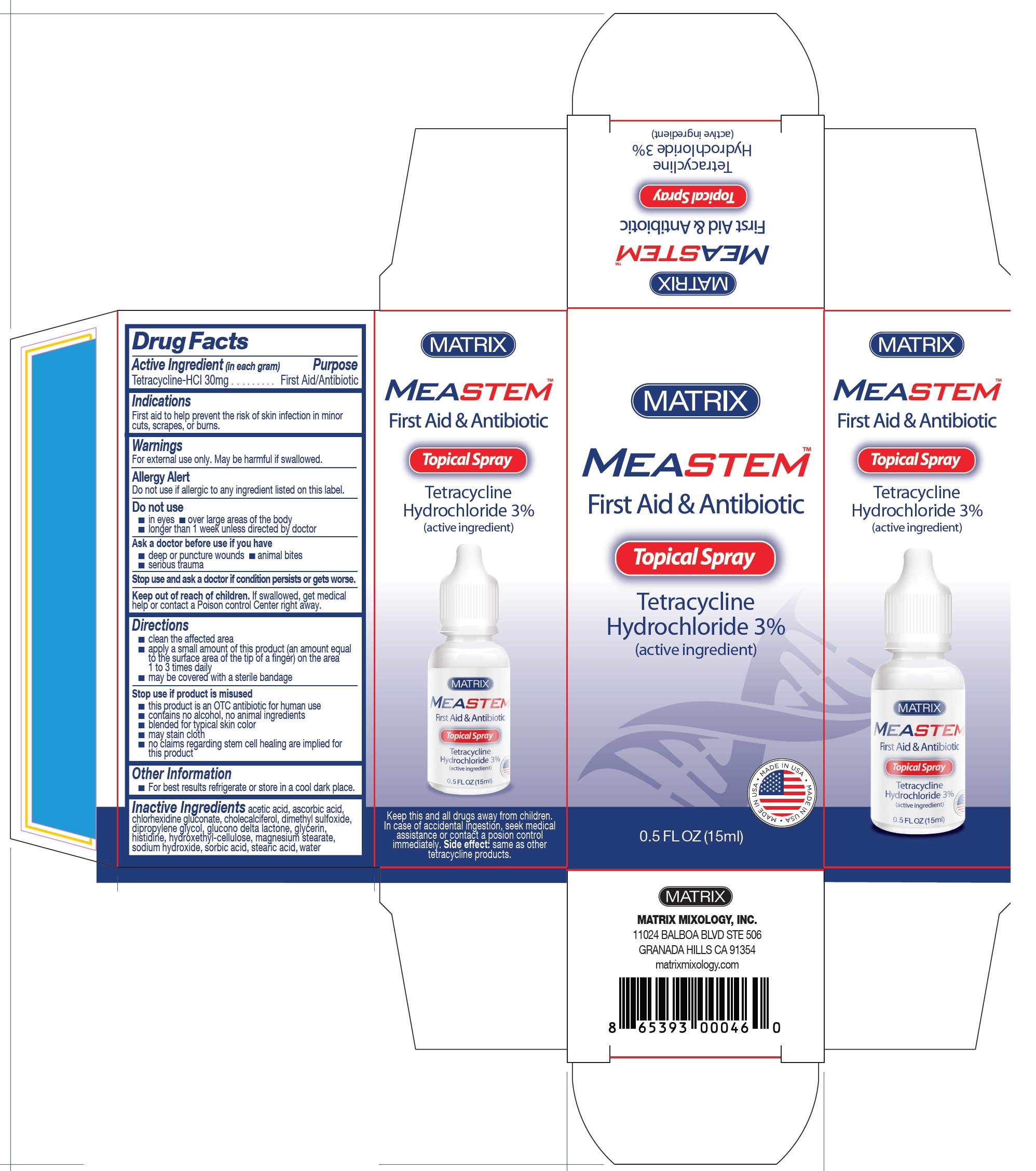
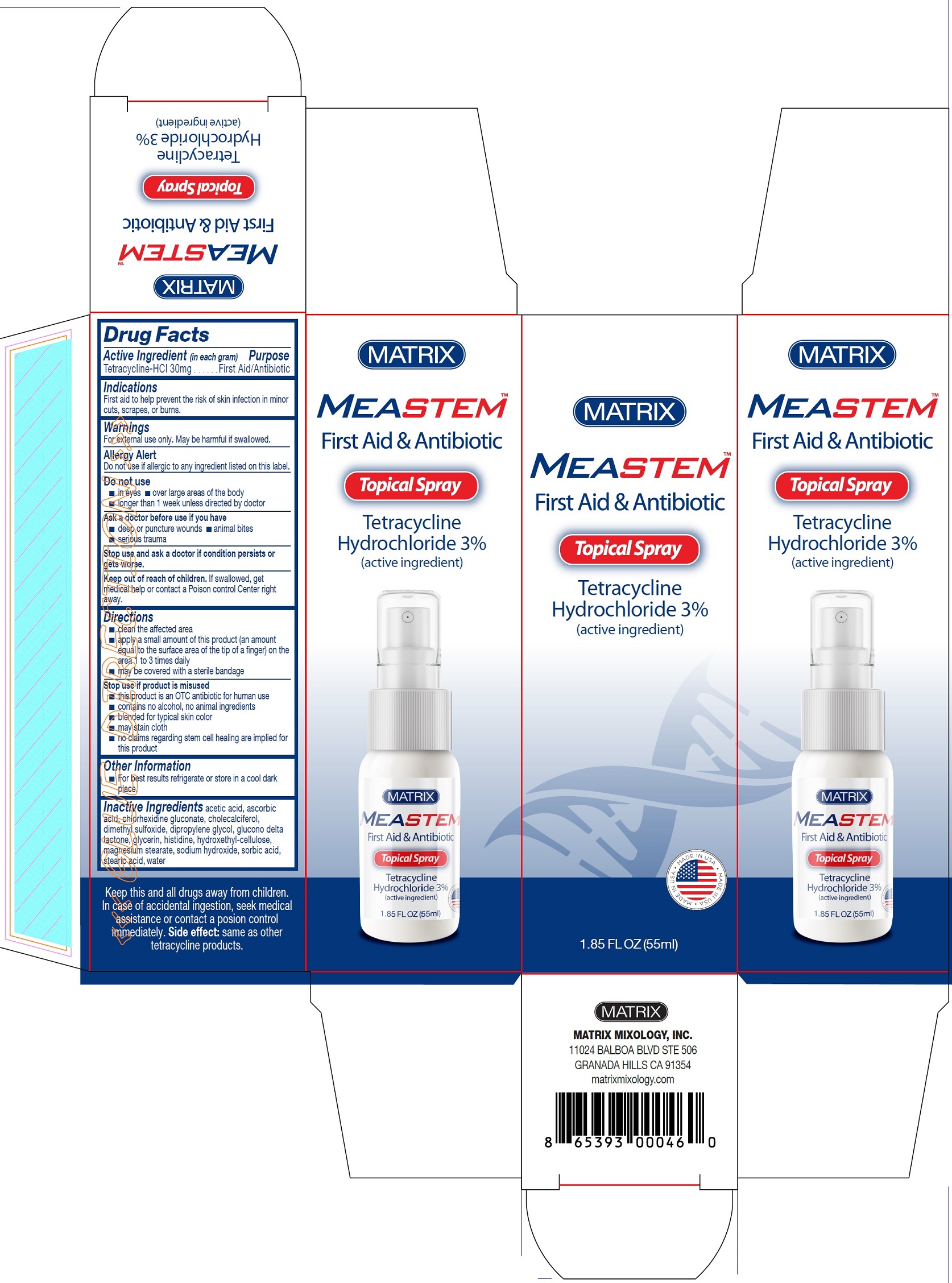
MeaStem First Aid & Antibiotic Spray
MeaStem First Aid and Antibiotic by
Drug Labeling and Warnings
MeaStem First Aid and Antibiotic by is a Otc medication manufactured, distributed, or labeled by MATRIX MIXOLOGY, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MEASTEM FIRST AID AND ANTIBIOTIC- tetracycline hydrochloride spray
MATRIX MIXOLOGY, INC
----------
MeaStem First Aid & Antibiotic Spray
Warnings
For external use only. May be harmful if swallowed.
AllergyAlert
Do not use if allergic to any ingredient listed on this label.
Directions
■ clean the affected area ■ apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily ■ may be covered with a sterile bandage
Stop use if product is misused
■ this product is an OTC antibiotic for human use ■ contains no alcohol, no animal ingredients ■ blended for typical skin color ■ may stain cloth ■ no claims regarding stem cell healing are implied for this product
| MEASTEM FIRST AID AND ANTIBIOTIC
tetracycline hydrochloride spray |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - MATRIX MIXOLOGY, INC (128885636) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.