Boroleum by Santus LLC Boroleum
Boroleum by
Drug Labeling and Warnings
Boroleum by is a Otc medication manufactured, distributed, or labeled by Santus LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
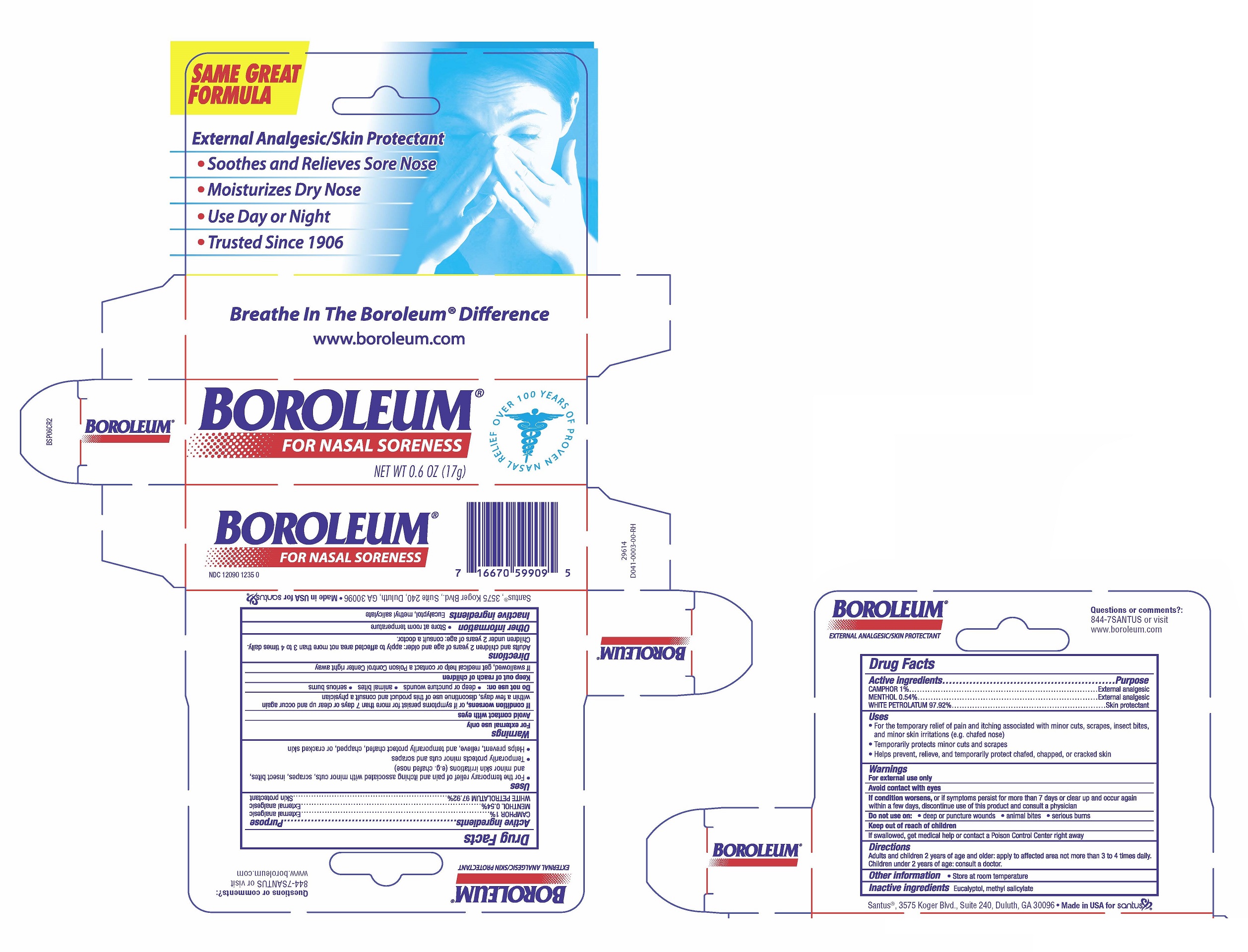
BOROLEUM FOR NASAL SORENESS- camphor, menthol, white petrolatum ointmentÂ
Santus LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Boroleum
Uses
- For the temporary relief of pain and itching associated with minor cuts, scrapes, insect bites, and minor skin irritations (e.g. chafed nose)
- Temporarily protects minor cuts and scrapes
- Helps prevent, relieve, and temporarily protect chafed, chapped, or cracked skin
Warnings
For external use only
Avoid contact with eyes
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a physician
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away
| BOROLEUMÂ
FOR NASAL SORENESS
camphor, menthol, white petrolatum ointment |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler -Â Santus LLC (079868223) |
Trademark Results [Boroleum]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BOROLEUM 87676134 5506884 Live/Registered |
Santus, LLC 2017-11-08 |
 BOROLEUM 71689245 0622573 Live/Registered |
SINCLAIR PHARMACAL CO., INC. 1955-06-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.