HAND-E-FOAM- otc antimicrobial drug products aerosol, foam
HAND-E-FOAM by
Drug Labeling and Warnings
HAND-E-FOAM by is a Otc medication manufactured, distributed, or labeled by DermaRite Industries, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
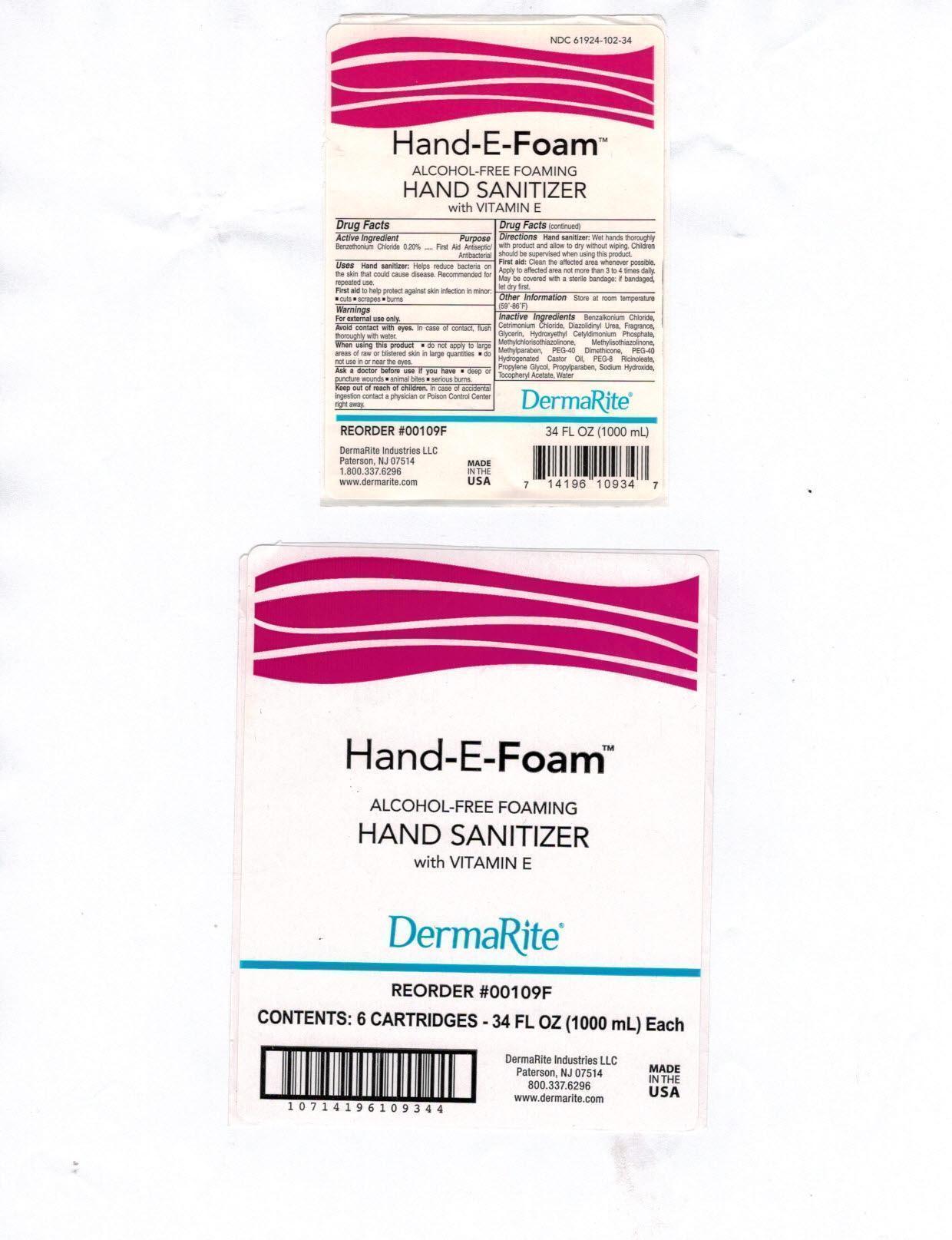
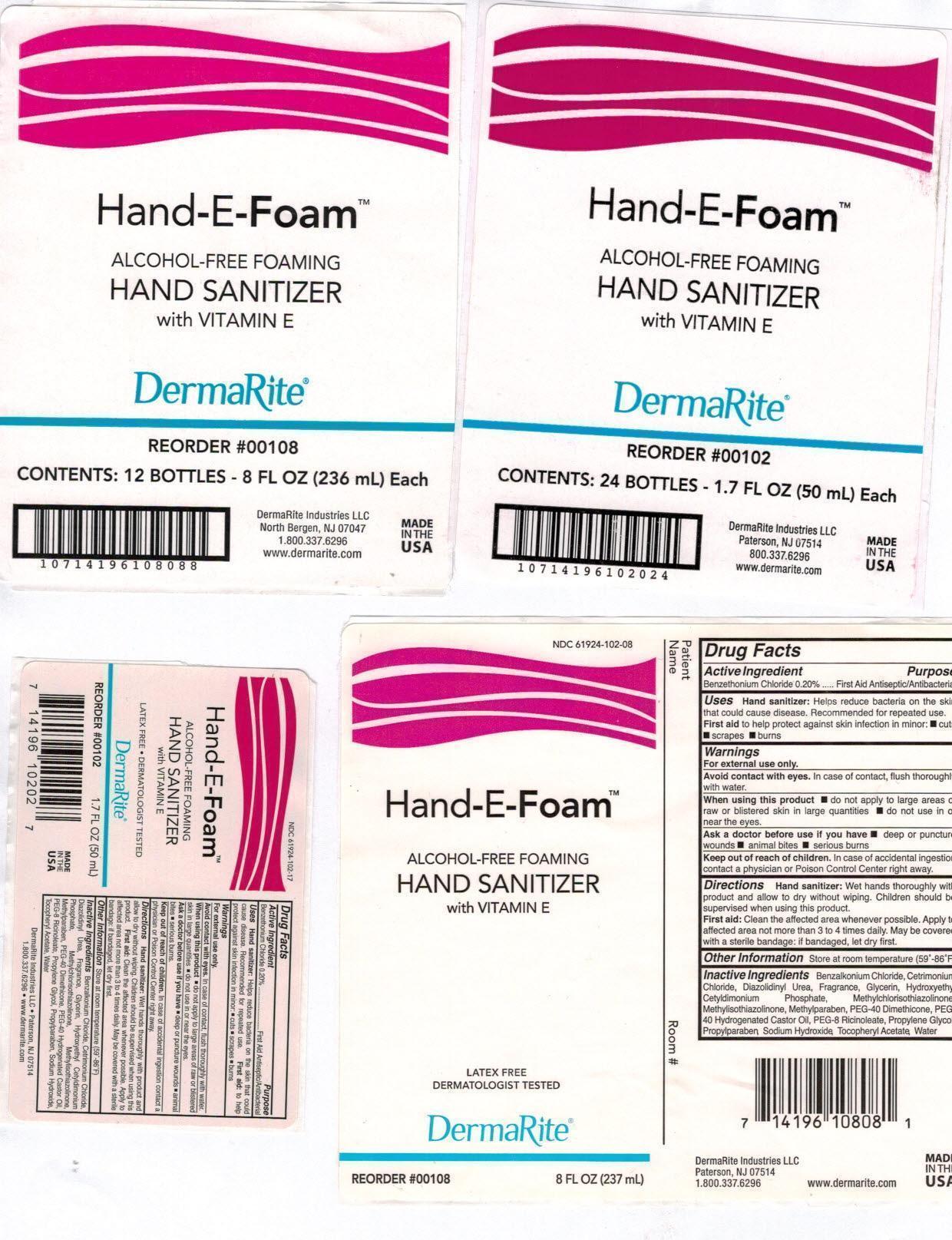
- Active Ingredient:
- Purpose:
- Uses:
-
Warnings:
- For external use only.
- Avoid contact with eyes. In case of contact, flush thoroughly with water.
- When using this product do not apply to large area of raw or blistered skin in large quantities; do not use in or near the eyes.
-
Ask a doctor before use if you have , deep of puncture wounds, animal bites, serious burns
- Warnings
-
Directions:
- Hand sanitizer: Wet hands thoroughly with product and allow to dry without wiping. Children should be supervised when using this product.
- First Aid: Clean the affected area whenever possible. Apply to affected area not more than 3 to 4 times daily. May be covered with a sterile bandage:if bandaged, let dry first.
- Other Information:
-
Inactive Ingredients:
Benzalkonium Chloride, Cetrimonium Chloride, Diazolidinyl Urea, Fragrance, Glycerin, Hydroxethyl Cetyldimonium Phosphate, Methylchloroisothiazolinone, Methylisothiazolinone, Methylparaben, PEG-40 Dimethicone, PEG-40 Hydrogenated Castor Oil, PEG-8 Ricinoleate, Propylene Glycol, Propylparaben, Sodium Hydroxide, Tocopheryl Acetate, Water
- Hand-E-Foam Package Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HAND-E-FOAM
otc antimicrobial drug products aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61924-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.002 g in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CETYLDIMONIUM PHOSPHATE (UNII: 9G05UO431K) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-8 DIMETHICONE (UNII: GIA7T764OD) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) PEG-8 RICINOLEATE (UNII: DM36F4D2OU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM HYDROXIDE (UNII: 55X04QC32I) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61924-102-08 237 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/08/2002 2 NDC: 61924-102-17 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/08/2002 3 NDC: 61924-102-34 1000 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 08/08/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/08/2002 Labeler - DermaRite Industries, LLC (883925562) Registrant - DermaRite Industries, LLC (883925562) Establishment Name Address ID/FEI Business Operations DermaRite Industries, LLC 883925562 manufacture(61924-102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.