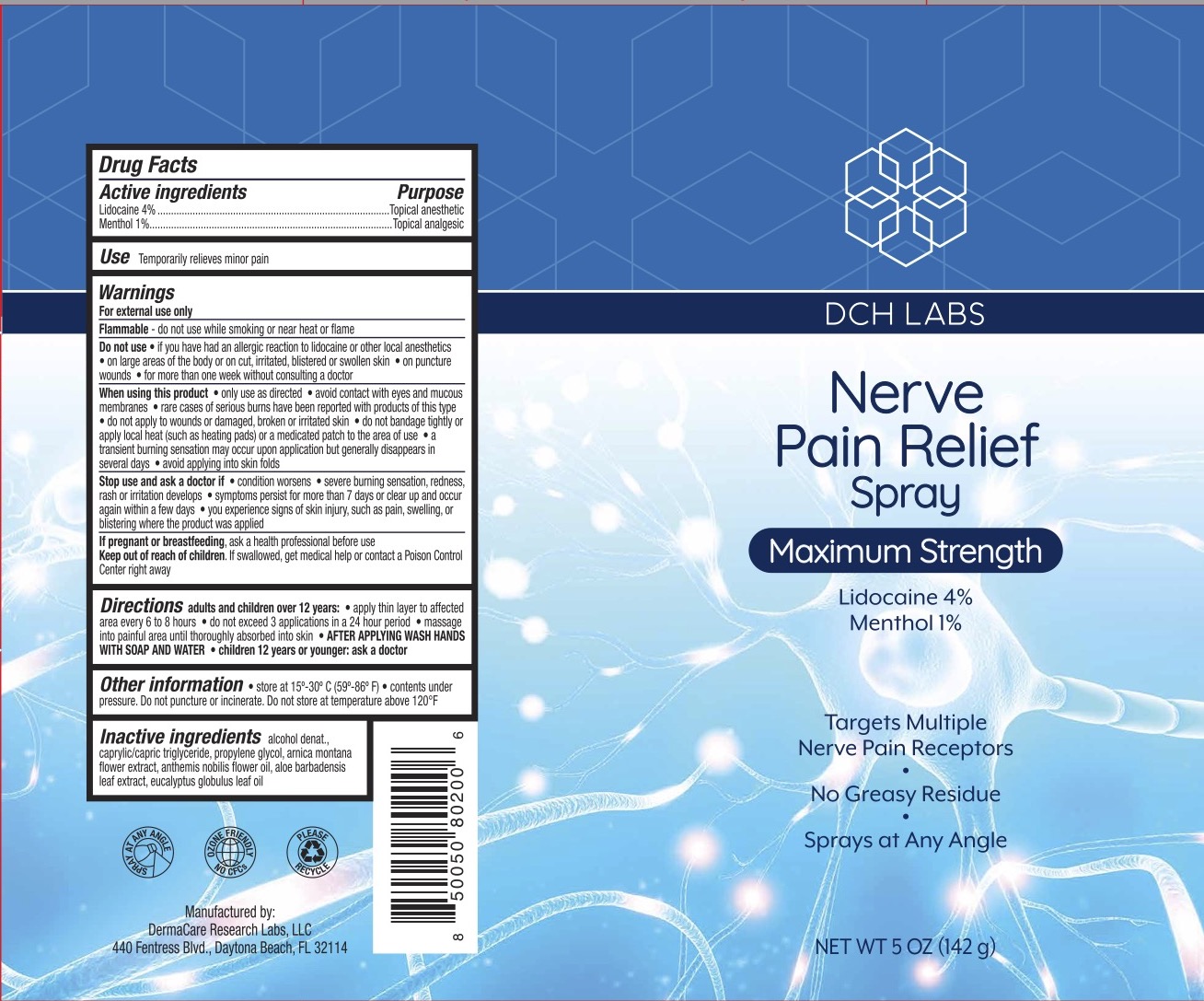
DCH NERVE PAIN RELIEF- lidocaine 4%, menthol 1% spray
DCH Nerve Pain Relief by
Drug Labeling and Warnings
DCH Nerve Pain Relief by is a Otc medication manufactured, distributed, or labeled by Derma Care Research Labs, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only.
Flammable--Keep away from fire or flame.
Do not use if you have had an allergic reaction to lidocaine or other local anesthetics, on large areas of he body or on cut, irritated, blistered or swollen skin, on puncture wounds, for more than one week without consulting a doctor.
When using this product only use as directed, avoid contact with eyes and mucous membranes, rare cases of serious burns have been reported with products of this type, do not apply to wounds or damaged, broken, or irritated skin, do not bandage tightly or apply local heat (such as heating pads) or a medicated path to the area of use, a transient burning sensation may occur upon application but generally disappears in several days, avoid applying into skin folds.
Stop use and ask a doctor if condition worsens, severe burning sensation, redness, rash or irritation develops, symptoms persist for more than 7 days or clear up and occur again within a few days, you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied.
.
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
When practical, cleanse the affected area with mild soap and warm water, Rinse thoroughly and gently pat dry. Spray affected area. Wash hands after applying. Adults and children 2 years of age and older, apply externally to the affected area up to 6 times a day. Children under 12 years of age: consult a doctor.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DCH NERVE PAIN RELIEF
lidocaine 4%, menthol 1% sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72839-223 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 g in 100 g Inactive Ingredients Ingredient Name Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALCOHOL (UNII: 3K9958V90M) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) EUCALYPTUS OIL (UNII: 2R04ONI662) ALOE VERA LEAF (UNII: ZY81Z83H0X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72839-223-05 142 g in 1 CAN; Type 0: Not a Combination Product 03/24/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/24/2023 Labeler - Derma Care Research Labs, LLC (116817470) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(72839-223)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.