ESZOPICLONE- eszopiclone tablet, film coated
Eszopiclone by
Drug Labeling and Warnings
Eszopiclone by is a Prescription medication manufactured, distributed, or labeled by Camber Pharmaceuticals, Inc., Annora Pharma Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ESZOPICLONE TABLETS safely and effectively. See full prescribing information for ESZOPICLONE TABLETS.
ESZOPICLONE tablets, for oral use, CIV
Initial U.S. Approval: 2004
WARNING: COMPLEX SLEEP BEHAVIOUR
See full prescribing information for complete boxed warning. Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of eszopiclone tablets. Some of these events may result in serious injuries, including death. Discontinue eszopiclone tablets immediately if a patient experiences a complex sleep behavior ( 4, 5.1).RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Eszopiclone tablets are indicated for the treatment of insomnia. Eszopiclone tablets has been shown to decrease sleep latency and improve sleep maintenance ( 1)
DOSAGE AND ADMINISTRATION
Use the lowest dose effective for the patient ( 2)
Recommended initial dose is 1 mg, immediately before bedtime, with at least 7 to 8 hours remaining before the planned time of awakening. May increase dose if clinically indicated, to a maximum of 3 mg ( 2.1)
Geriatric or debilitated patients: Dose should not exceed 2 mg ( 2.2)
Patients with severe hepatic impairment, or taking potent CYP3A4 inhibitors: Dose should not exceed 2 mg ( 2.3)
Do not take with or immediately after a meal ( 2.5)DOSAGE FORMS AND STRENGTHS
Tablets: 1 mg, 2 mg, and 3 mg ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
CNS Depressant Effects Impaired alertness and motor coordination, including risk of morning impairment. Risk increases with dose and use with other CNS depressants and alcohol. Caution patients taking 3 mg dose against driving and against activities requiring complete mental alertness during the morning after use. ( 5.2)
Evaluate for Comborbid DiagnosesReevaluate if insomnia persists after 7 to 10 days of use ( 5.3)
Severe Anaphylactic/Anaphylactoid Reactions(angioedema and anaphylaxis have been reported): Do not rechallenge if such reactions occur ( 5.4)
Abnormal Thinking amd Behavioral Changes including decreased inhibition, bizarre behavior, agitation and depersonalization have been reported. Immediately evaluate any new onset of behavioral changes ( 5.5)
Worsening of Depression or Suicidal Thinkingmay occur: Prescribe the least number of tablets feasible to avoid intentional overdose ( 5.5, 5.8)
Withdrawal EffectsSymptoms may occur with rapid dose reduction or discontinuation ( 5.6, 9.3)
Elderly PatientsUse lower dose due to impaired motor, cognitive performance and increased sensitivity ( 2.2, 5.8)
Patients with Hepatic Impairment, Impaired Respiratory Function, Impaired Drug Metabolism or Hemodynamic ResponsesUse with caution ( 5.8)ADVERSE REACTIONS
Most commonly observed adverse reactions (incidence ≥2%) were unpleasant taste, headache, somnolence, respiratory infection, dizziness, dry mouth, rash, anxiety, hallucinations, and viral infections ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Annora Pharma Private Limited at 1-866-495-1995 or FDA at 1-800-FDA-1088 or ww.fda.gov/medwatch.
DRUG INTERACTIONS
CNS DepressantsAdditive CNS-depressant effects with combination use. Use with ethanol causes additive psychomotor impairment ( 7.1)
RifampicinCombination use may decrease exposure and effects of eszopiclone tablets ( 7.2)
KetoconazoleCombination use increases exposure and effect of eszopiclone tablets. Dose reduction of eszopiclone tablet is needed ( 7.2)USE IN SPECIFIC POPULATIONS
Pediatric UseSafety and effectiveness not established. Dizziness, dysgeusia, hallucinations, suicidal ideation reported ( 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: COMPLEX SLEEP BEHAVIORS
1 INDICATIONS & USAGE
2 DOSAGE & ADMINISTRATION
2.1 Dosage in Adults
2.2 Geriatric or Debilitated Patients
2.3 Patients with Severe Hepatic Impairment, or Taking Potent CYP3A4 Inhibitors
2.4 Use with CNS Depressants
2.5 Administration with Food
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Complex Sleep Behaviours
5.2 CNS Depressant Effects and Next-Day Impairment
5.3 Need to Evaluate for Comborbid Diagnoses
5.4 Severe Anaphylactic and Anaphylactoid Reactions
5.5 Abnormal Thinking and Behavioral Changes
5.6 Withdrawal Effects
5.7 Timing of Drug Administration
5.8 Special Populations
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CNS Active Drugs
7.2 Drugs that Inhibit or Induce CYP3A4
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
9.4 Tolerance
10 OVERDOSAGE
10.1 Signs and Symptoms
10.2 Recommended Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
14 CLINICAL STUDIES
14.1 Transient Insomnia
14.2 Chronic Insomnia (Adults and Elderly)
14.3 Studies Pertinent to Safety Concerns for Sedative Hypnotic Drugs
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: COMPLEX SLEEP BEHAVIORS
WARNING: COMPLEX SLEEP BEHAVIORS Complex sleep behaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following use of eszopiclone tablets. Some of these events may result in serious injuries, including death. Discontinue eszopiclone tablets immediately if a patient experiences a complex sleep behavior [see Contraindications (4)and Warnings and Precautions (5.1)].
-
1 INDICATIONS & USAGE
Eszopiclone tablets are indicated for the treatment of insomnia. In controlled outpatient and sleep laboratory studies, eszopiclone tablets administered at bedtime decreased sleep latency and improved sleep maintenance.
The clinical trials performed in support of efficacy were up to 6 months in duration. The final formal assessments of sleep latency and maintenance were performed at 4 weeks in the 6-week study (adults only), at the end of both 2-week studies (elderly only) and at the end of the 6-month study (adults only). -
2 DOSAGE & ADMINISTRATION
Use the lowest effective dose for the patient.
2.1 Dosage in Adults
The recommended starting dose is 1 mg. Dosing can be raised to 2 mg or 3 mg if clinically indicated. In some patients, the higher morning blood levels of eszopiclone tablets following use of the 2 mg or 3 mg dose increase the risk of next day impairment of driving and other activities that require full alertness [ see Warnings and Precautions (5.1)]. The total dose of eszopiclone tablets should not exceed 3 mg, once daily immediately before bedtime [ see Warnings and Precautions (5.6)].
2.2 Geriatric or Debilitated Patients
The total dose of eszopiclone tablets should not exceed 2 mg in elderly or debilitated patients.
2.3 Patients with Severe Hepatic Impairment, or Taking Potent CYP3A4 Inhibitors
In patients with severe hepatic impairment, or in patients coadministered eszopiclone tablets with potent CYP3A4 inhibitors, the total dose of eszopiclone tablets should not exceed 2 mg [ see Warnings and Precautions (5.7)].
2.4 Use with CNS Depressants
Dosage adjustments may be necessary when eszopiclone tablets are combined with othercentral nervous system (CNS) depressant drugs because of the potentially additive effects [ see Warnings and Precautions (5.1)].
2.5 Administration with Food
Taking eszopiclone tablets with or immediately after a heavy, high-fat meal results in slower absorption and would be expected to reduce the effect of eszopiclone tablets on sleep latency [see Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS & STRENGTHS
Eszopiclone tablets, USP are available in 1 mg, 2 mg and 3 mg strengths for oral administration.
Eszopiclone tablets USP, 3 mg are dark blue to blue, round, biconvex, film-coated tablets debossed with ‘H’ on one side and ‘E16’ on the other side.
Eszopiclone tablets USP, 2 mg are white to off-white, round, biconvex, film-coated tablets debossed with ‘H’ on one side and ‘E15’ on the other side.
Eszopiclone tablets USP, 1 mg are light blue, round, biconvex, film-coated tablets debossed with ‘H’ on one side and ‘E14’ on the other side. -
4 CONTRAINDICATIONS
Eszopiclonetablet is contraindicated in patients who have experienced complex sleep behaviors after taking eszopiclone tablets [see Warnings and Precautions (5.1)].
Eszopiclone tablet is contraindicated in patients with known hypersensitivity to eszopiclone. Hypersensitivity reactions include anaphylaxis and angioedema [see Warnings and Precautions (5.3)]. -
5 WARNINGS AND PRECAUTIONS
5.1 Complex Sleep Behaviours
Complex sleepbehaviors including sleep-walking, sleep-driving, and engaging in other activities while not fully awake may occur following the first or any subsequent use of eszopiclone tablet. Patients can be seriously injured or injure others during complex sleep behaviors. Such injuries may result in a fatal outcome. Other complex sleep behaviors (e.g., preparing and eating food, making phone calls, or having sex) have also been reported. Patients usually do not remember these events. Post-marketing reports have shown that complex sleep behaviors may occur with eszopiclone alone at recommended dosages, with or without the concomitant use of alcohol or other CNS depressants [see Drug Interactions (7.1)]. Discontinue eszopiclone immediately if a patient experiences a complex sleep behavior.
5.2 CNS Depressant Effects and Next-Day Impairment
Eszopiclone tablet is a CNS depressant and can impair daytime function in some patients at the higher doses (2 mg or 3 mg), even when used as prescribed. Prescribers should monitor for excess depressant effects, but impairment can occur in the absence of symptoms (or even with subjective improvement), and impairment may not be reliably detected by ordinary clinical exam (i.e., less than formal psychomotor testing). While pharmacodynamic tolerance or adaptation to some adverse depressant effects of eszopiclone tablets may develop, patients using 3 mg eszopiclone tablets should be cautioned against driving or engaging in other hazardous activities or activities requiring complete mental alertness the day after use.
Additive effects occur with concomitant use of other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants, alcohol), including daytime use. Downward dose adjustment of eszopiclone tablets and concomitant CNS depressants should be considered [see Dosage and Administration (2.4)].
The use of eszopiclone tablets with other sedative-hypnotics at bedtime or the middle of the night is not recommended.
The risk of next-day psychomotor impairment is increased if eszopiclone tablets are taken with less than a full night of sleep remaining (7- to 8 hours); if higher than the recommended dose is taken; if co-administered with other CNS depressants; or coadministered with other drugs that increase the blood levels of eszopiclone [see Dosage and Administration (2.3) and Clinical Studies (14.3)].
Becauseeszopiclone tablets can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
5.3 Need to Evaluate for Comborbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated.Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including eszopiclone tablets. Because some of the important adverse effects of eszopiclone tablets appear to be dose related, it is important to use the lowest possible effective dose, especially in the elderly [see Dosage and Administration (2.1)].
5.4 Severe Anaphylactic and Anaphylactoid Reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including eszopiclone tablets. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with eszopiclone tablets should not be rechallenged with the drug.
5.5 Abnormal Thinking and Behavioral Changes
A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), similar to effects produced by alcohol and other CNS depressants. Other reported behavioral changes have included bizarre behavior, agitation, hallucinations, and depersonalization. Amnesia and other neuropsychiatric symptoms may occur unpredictably.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.5.6 Withdrawal Effects
Following rapid dose decrease or abrupt discontinuation of the use of sedative/hypnotics, there have been reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs [see Drug Abuse and Dependence (9)].
5.7 Timing of Drug Administration
Eszopiclone tablets should be taken immediately before bedtime. Taking a sedative/hypnotic while still up and about may result in short-term memory impairment, hallucinations, impaired coordination, dizziness, and lightheadedness.
5.8 Special Populations
Use in Elderly and/or Debilitated Patients
Impaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. The dose should not exceed 2 mg in elderly or debilitated patients [see Dosage and Administration (2.2)].
Use in Patients with Concomitant Illness
Clinical experience with eszopiclone in patients with concomitant illness is limited. Eszopiclone tablets should be used with caution in patients with diseases or conditions that could affect metabolism or hemodynamic responses.
A study in healthy volunteers did not reveal respiratory-depressant effects at doses 2.5-fold higher (7 mg) than the recommended dose of eszopiclone. Caution is advised, however, if eszopiclone tablets are prescribed to patients with compromised respiratory function.
The dose of eszopiclone tablets should not exceed 2 mg in patients with severe hepatic impairment, because systemic exposure is doubled in such subjects. No dose adjustment appears necessary for subjects with mild or moderate hepatic impairment. No dose adjustment appears necessary in subjects with any degree of renal impairment, since less than 10% of eszopiclone is excreted unchanged in the urine.
The dose of eszopiclone tablets should be reduced in patients who are administered potent inhibitors of CYP3A4, such as ketoconazole, while taking eszopiclone tablets. Downward dose adjustment is also recommended when eszopiclone tablets are administered with agents having known CNS-depressant effects.
Use in Patients with Depression
In primarily depressed patients treated with sedative-hypnotics, worsening of depression, including suicidal thoughts and actions (including completed suicides), have been reported in association with the use of sedative/hypnotics.
Sedative/hypnotic drugs should be administered with caution to patients exhibiting signs and symptoms of depression. Suicidal tendencies may be present in such patients, and protective measures may be required. Intentional overdose is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time. -
6 ADVERSE REACTIONS
The following are described in more detail in the Warnings and Precautionssection of the label:
Complex Sleep Behaviors [see Boxed Warning and Warnings and Precautions (5.1)]
CNS Depressant Effects and Next-Day Impairment [see Warnings and Precautions (5.2)]
Need to Evaluate for Comorbid Diagnoses [see Warnings and Precautions (5.3)]
Severe Anaphylactic and Anaphylactoid Reactions [see Warnings and Precautions (5.4)]
Abnormal Thinking and Behavioral Changes [see Warnings and Precautions (5.5)]
Withdrawal Effects [see Warnings and Precautions (5.6)]
Timing of Drug Administration [see Warnings and Precautions (5.7)]
Special Populations [see Warnings and Precautions (5.8)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The premarketing development program for eszopiclone tablets included eszopiclone exposures in patients and/or normal subjects from two different groups of studies: approximately 400 normal subjects in clinical pharmacology/pharmacokinetic studies, and approximately 1550 patients in placebo-controlled clinical effectiveness studies, corresponding to approximately 263 patient-exposure years. The conditions and duration of treatment with eszopiclone tablets varied greatly and included (in overlapping categories) open-label and double-blind phases of studies, inpatients and outpatients, and short-term and longer-term exposure. Adverse reactions were assessed by collecting adverse events, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, adverse reaction of the type listed. A reaction was considered treatment-emergent if it occurred for the first time or worsened while the patient was receiving therapy following baseline evaluation.6.1 Clinical Trials Experience
Adverse Reactions Resulting in Discontinuation of Treatment
In placebo-controlled, parallel-group clinical trials in the elderly, 3.8% of 208 patients who received placebo, 2.3% of 215 patients who received 2 mg eszopiclone tablets, and 1.4% of 72 patients who received 1 mg eszopiclone tablets discontinued treatment due to an adverse reaction. In the 6-week parallel-group study in adults, no patients in the 3 mg arm discontinued because of an adverse reaction. In the long-term 6-month study in adult insomnia patients, 7.2% of 195 patients who received placebo and 12.8% of 593 patients who received 3 mg eszopiclone tablets discontinued due to an adverse reaction. No reaction that resulted in discontinuation occurred at a rate of greater than 2%.
Adverse Reactions Observed at an Incidence of ≥2% in Controlled Trials
Table 1 shows the incidence of adverse reactions from a Phase 3 placebo-controlled study of eszopiclone tablets at doses of 2 or 3 mg in nonelderly adults. Treatment duration in this trial was 44 days. The table includes only reactions that occurred in 2% or more of patients treated with eszopiclone tablets 2 mg or 3 mg in which the incidence in patients treated with eszopiclone was greater than the incidence in placebo-treated patients.
Table 1: Incidence (%) of Adverse Reactions in a 6-Week Placebo-Controlled Study in Nonelderly Adults with Eszopiclone Tablets 1Adverse Reaction
Placebo
(n=99)
Eszopiclone tablets 2 mg (n=104)
Eszopiclone tablets 3 mg (n=105)
Body as a Whole
Headache
13
21
17
Viral Infection
1
3
3
Digestive System
Dry Mouth
3
5
7
Dyspepsia
4
4
5
Nausea
4
5
4
Vomiting
1
3
0
Nervous System
Anxiety
0
3
1
Confusion
0
0
3
Depression
0
4
1
Dizziness
4
5
7
Hallucinations
0
1
3
Libido Decreased
0
0
3
Nervousness
3
5
0
Somnolence
3
10
8
Respiratory System
Infection
3
5
10
Skin and Appendages
Rash
1
3
4
Special Senses
Unpleasant Taste
3
17
34
Urogenital System
Dysmenorrhea *
0
3
0
Gynecomastia **
0
3
0
1Reactions for which the eszopiclone tablets incidence was equal to or less than placebo are not listed on the table, but included the following: abnormal dreams, accidental injury, back pain, diarrhea, flu syndrome, myalgia, pain, pharyngitis, and rhinitis.
* Gender-specific adverse reaction in females
** Gender-specific adverse reaction in males
Adverse reactions from Table 1 that suggest a dose-response relationship in adults include viral infection, dry mouth, dizziness, hallucinations, infection, rash, and unpleasant taste, with this relationship clearest for unpleasant taste.
Table 2 shows the incidence of adverse reactions from combined Phase 3 placebo-controlled studies of eszopiclone tablets at doses of 1 or 2 mg in elderly adults (ages 65 to 86). Treatment duration in these trials was 14 days. The table includes only reactions that occurred in 2% or more of patients treated with eszopiclone tablets 1 mg or 2 mg in which the incidence in patients treated with eszopiclone tablets was greater than the incidence in placebo-treated patients.
Table 2: Incidence (%) of Adverse Reactions in Elderly Adults (Ages 65 to 86 Years) in 2-Week Placebo-Controlled Trials with Eszopiclone Tablets 1
Adverse Reactions
Placebo (n=208)
Eszopiclone tablets
1 mg
(n=72)
Eszopiclone tablets
2 mg
(n=215)
Body as a Whole
Accidental Injury
1
0
3
Headache
14
15
13
Pain
2
4
5
Digestive System
Diarrhea
2
4
2
Dry Mouth
2
3
7
Dyspepsia
2
6
2
Nervous System
Abnormal Dreams
0
3
1
Dizziness
2
1
6
Nervousness
1
0
2
Neuralgia
0
3
0
Skin and Appendages
Pruritus
1
4
1
Special Senses
Unpleasant Taste
0
8
12
Urogenital System
Urinary Tract Infection
0
3
0
1 Reactions for which the eszopiclone tablets incidence was equal to or less than placebo are not listed on the table, but included the following: abdominal pain, asthenia, nausea, rash, and somnolence.
Adverse reactions from Table 2 that suggest a dose-response relationship in elderly adults include pain, dry mouth, and unpleasant taste, with this relationship again clearest for unpleasant taste.
These figures cannot be used to predict the incidence of adverse reactions in the course of usual medical practice because patient characteristics and other factors may differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contributions of drug and nondrug factors to the adverse reaction incidence rate in the population studied.
Other Reactions Observed During the Premarketing Evaluation of Eszopiclone Tablets
Following is a list of modified COSTART terms that reflect adverse reactions as defined in the introduction to the Adverse Reactionssection and reported by approximately 1550 subjects treated with eszopiclone tablets at doses in the range of 1 to 3.5 mg/day during Phase 2 and 3 clinical trials throughout the United States and Canada. All reported reactions are included except those already listed in Tables 1 and 2 or elsewhere in labeling, minor reactions common in the general population, and reactions unlikely to be drug-related. Although the reactions reported occurred during treatment with eszopiclone tablets, they were not necessarily caused by it.
Reactions are further categorized by body system and listed in order of decreasing frequency according to the following definitions: frequentadverse reactions are those that occurred on one or more occasions in at least 1/100 patients; infrequentadverse reactions are those that occurred in fewer than 1/100 patients but in at least 1/1,000 patients; rareadverse reactions are those that occurred in fewer than 1/1,000 patients. Gender-specific reactions are categorized based on their incidence for the appropriate gender.
Body as a Whole: Frequent:chest pain; Infrequent:allergic reaction, cellulitis, face edema, fever, halitosis, heat stroke, hernia, malaise, neck rigidity, photosensitivity.
Cardiovascular System: Frequent:migraine; Infrequent:hypertension; Rare:thrombophlebitis.
Digestive System: Infrequent:anorexia, cholelithiasis, increased appetite, melena, mouth ulceration, thirst, ulcerative stomatitis; Rare:colitis, dysphagia, gastritis, hepatitis, hepatomegaly, liver damage, stomach ulcer, stomatitis, tongue edema, rectal hemorrhage.
Hemic and Lymphatic System: Infrequent:anemia, lymphadenopathy.
Metabolic and Nutritional: Frequent:peripheral edema; Infrequent:hypercholesteremia, weight gain, weight loss; Rare:dehydration, gout, hyperlipemia, hypokalemia.
Musculoskeletal System: Infrequent:arthritis, bursitis, joint disorder (mainly swelling, stiffness, and pain), leg cramps, myasthenia, twitching; Rare:arthrosis, myopathy, ptosis.
Nervous System: Infrequent:agitation, apathy, ataxia, emotional lability, hostility, hypertonia, hypesthesia, incoordination, insomnia, memory impairment, neurosis, nystagmus, paresthesia, reflexes decreased, thinking abnormal (mainly difficulty concentrating), vertigo; Rare:abnormal gait, euphoria, hyperesthesia, hypokinesia, neuritis, neuropathy, stupor, tremor.
Respiratory System: Infrequent:asthma, bronchitis, dyspnea, epistaxis, hiccup, laryngitis.
Skin and Appendages: Infrequent:acne, alopecia, contact dermatitis, dry skin, eczema, skin discoloration, sweating, urticaria; Rare:erythema multiforme, furunculosis, herpes zoster, hirsutism, maculopapular rash, vesiculobullous rash.
Special Senses: Infrequent:conjunctivitis, dry eyes, ear pain, otitis externa, otitis media, tinnitus, vestibular disorder; Rare:hyperacusis, iritis, mydriasis, photophobia.
Urogenital System: Infrequent:amenorrhea, breast engorgement, breast enlargement, breast neoplasm, breast pain, cystitis, dysuria, female lactation, hematuria, kidney calculus, kidney pain, mastitis, menorrhagia, metrorrhagia, urinary frequency, urinary incontinence, uterine hemorrhage, vaginal hemorrhage, vaginitis; Rare:oliguria, pyelonephritis, urethritis.6.2 Postmarketing Experience
In addition to the adverse reactions observed during clinical trials, dysosmia, an olfactory dysfunction that is characterized by distortion of the sense of smell, has been reported during postmarketing surveillance with eszopiclone tablets. Because this event is reported spontaneously from a population of unknown size, it is not possible to estimate the frequency of this event.
-
7 DRUG INTERACTIONS
7.1 CNS Active Drugs
Ethanol:An additive effect on psychomotor performance was seen with coadministration of eszopiclone and ethanol [see Warnings and Precautions (5.1, 5.2)].
Olanzapine:Coadministration of eszopiclone and olanzapine produced a decrease in DSST scores. The interaction was pharmacodynamic; there was no alteration in the pharmacokinetics of either drug.7.2 Drugs that Inhibit or Induce CYP3A4
Drugs that Inhibit CYP3A4 (Ketoconazole)
CYP3A4 is a major metabolic pathway for elimination of eszopiclone. The exposure of eszopiclone was increased by coadministration of ketoconazole, a potent inhibitor of CYP3A4. Other strong inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, nefazodone, troleandomycin, ritonavir, nelfinavir) would be expected to behave similarly. Dose reduction of eszopiclone tablet is needed for patient co-administered eszopiclone tablets with potent CYP3A4 inhibitors [see Dosage and Administration (2.3)] .
Drugs that Induce CYP3A4 (Rifampicin)
Racemic zopiclone exposure was decreased 80% by concomitant use of rifampicin, a potent inducer of CYP3A4. A similar effect would be expected with eszopiclone. Combination use with CYP3A4 inducer may decrease the exposure and effects of eszopiclone tablets. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available pharmacovigilance data with eszopiclone use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies conducted in pregnant rats and rabbits throughout organogenesis, there was no evidence of teratogenicity. Administration of eszopiclone to rats throughout pregnancy and lactation resulted in offspring toxicities at all doses tested; the lowest dose was approximately 200 times the maximum recommended human dose (MRHD) of 3 mg/day based on mg/m 2body surface area (See Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Oral administration of eszopiclone to pregnant rats (62.5, 125, or 250 mg/kg/day) and rabbits (4, 8, or 16 mg/kg/day) throughout organogenesis showed no evidence of teratogenicity up to the highest doses tested. In rats, reduced fetal weight and increased incidences of skeletal variations and/or delayed ossification were observed at the mid and high doses. The no-observed-effect dose for adverse effects on embryofetal development is 200 times the maximum recommended human dose (MRHD) of 3 mg/day on a mg/m 2basis. No effects on embryofetal development were observed in rabbits; the highest dose tested is approximately 100 times the MRHD on a mg/m 2basis.
Oral administration of eszopiclone (60, 120, or 180 mg/kg/day) to pregnant rats throughout the pregnancy and lactation resulted in increased post-implantation loss, decreased postnatal pup weights and survival, and increased pup startle response at all doses. The lowest dose tested is approximately 200 times the MRHD on a mg/m 2basis. Eszopiclone had no effects on other developmental measures or reproductive function in the offspring.8.2 Lactation
Risk Summary
There are no data on the presence of eszopiclone in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for eszopiclone and any potential adverse effects on the breastfed infant from eszopiclone or from the underlying maternal condition.8.4 Pediatric Use
Safety and effectiveness of eszopiclone tablets have not been established in pediatric patients.
Eszopiclone tablets failed to demonstrate efficacy in controlled clinical studies of pediatric patients with Attention-Deficit/Hyperactivity (ADHD) associated insomnia.
In a 12–week controlled study, 483 pediatric patients (aged 6 to17 years) with insomnia associated with ADHD (with 65% of the patients using concomitant ADHD treatments) were treated with oral tablets of eszopiclone (1, 2 or 3 mg tablets, n=323), or placebo (n=160). Eszopiclone did not significantly decrease latency to persistent sleep, compared to placebo, as measured by polysomnography after 12 weeks of treatment. Psychiatric and nervous system disorders comprised the most frequent treatment-emergent adverse reactions observed with eszopiclone versus placebo and included dysgeusia (9% vs.1%), dizziness (6% vs. 2%), hallucinations (2% vs. 0%) and suicidal ideation (0.3% vs. 0%). Nine patients on eszopiclone (3%) discontinued treatment due to an adverse reaction compared to 3 patients on placebo (2%).
In studies in which eszopiclone (2 to 300 mg/kg/day) was orally administered to young rats from weaning through sexual maturity, neurobehavioral impairment (altered auditory startle response) and reproductive toxicity (adverse effects on male reproductive organ weights and histopathology) were observed at doses ≥5 mg/kg/day. Delayed sexual maturation was noted in males and females at ≥10 mg/kg/day. The no-effect dose (2 mg/kg) was associated with plasma exposures (AUC) for eszopiclone and metabolite (S)-desmethylzopiclone [(S)-DMZ] approximately 2 times plasma exposures in humans at the MRHD in adults (3 mg/day).
When eszopiclone (doses from 1 to 50 mg/kg/day) was orally administered to young dogs from weaning through sexual maturity, neurotoxicity (convulsions) was observed at doses ≥ 5 mg/kg/day. Hepatotoxicity (elevated liver enzymes and hepatocellular vacuolation and degeneration) and reproductive toxicity (adverse effects on male reproductive organ weights and histopathology) were noted at dose ≥10 mg/kg/day. The no-effect dose (1 mg/kg) was associated with plasma exposures (AUC) to eszopiclone and (S)-DMZ approximately 3 and 2 times, respectively, plasma exposures in humans at the MRHD in adults.8.5 Geriatric Use
A total of 287 subjects in double-blind, parallel-group, placebo-controlled clinical trials who received eszopiclone were 65 to 86 years of age. The overall pattern of adverse events for elderly subjects (median age = 71 years) in 2-week studies with nighttime dosing of 2 mg eszopiclone was not different from that seen in younger adults [see Adverse Reactions (6)]. Eszopiclone tablets 2 mg exhibited significant reduction in sleep latency and improvement in sleep maintenance in the elderly population. Compared with nonelderly adults, subjects 65 years and older had longer elimination and higher total exposure to eszopiclone. Therefore, dose reduction is recommended in elderly patients [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)] .
8.6 Hepatic Impairment
No dose adjustment is necessary for patients with mild-to-moderate hepatic impairment. Exposure was increased in severely impaired patients compared with healthy volunteers. The dose of eszopiclone tablets should not exceed 2 mg in patients with severe hepatic impairment. Eszopiclone tablets should be used with caution in patients with hepatic impairment [see Dosage and Administration (2.3), Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Eszopiclone tablet is a Schedule IV controlled substance under the Controlled Substances Act. Other substances under the same classification are benzodiazepines and the nonbenzodiazepine hypnotics zaleplon and zolpidem. While eszopiclone is a hypnotic agent with a chemical structure unrelated to benzodiazepines, it shares some of the pharmacologic properties of the benzodiazepines.
9.2 Abuse
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for nonmedical purposes, often in combination with other psychoactive substances. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
In a study of abuse liability conducted in individuals with known histories of benzodiazepine abuse, eszopiclone at doses of 6 and 12 mg produced euphoric effects similar to those of diazepam 20 mg. In this study, at doses 2-fold or greater than the maximum recommended doses, a dose-related increase in reports of amnesia and hallucinations was observed for both eszopiclone tablets and diazepam.9.3 Dependence
The clinical trial experience with eszopiclone tablets revealed no evidence of a serious withdrawal syndrome. Nevertheless, the following adverse events included in DSM-IV criteria for uncomplicated sedative/hypnotic withdrawal were reported during clinical trials following placebo substitution occurring within 48 hours following the last eszopiclone tablets treatment: anxiety, abnormal dreams, nausea, and upset stomach. These reported adverse events occurred at an incidence of 2% or less. Use of benzodiazepines and similar agents may lead to physical and psychological dependence. The risk of abuse and dependence increases with the dose and duration of treatment and concomitant use of other psychoactive drugs. The risk is also greater for patients who have a history of alcohol or drug abuse or history of psychiatric disorders. These patients should be under careful surveillance when receiving eszopiclone tablets or any other hypnotic.
9.4 Tolerance
Some loss of efficacy to the hypnotic effect of benzodiazepines and benzodiazepine-like agents may develop after repeated use of these drugs for a few weeks.
No development of tolerance to any parameter of sleep measurement was observed over six months. Tolerance to the efficacy of eszopiclone tablets 3 mg was assessed by 4-week objective and 6-week subjective measurements of time to sleep onset and sleep maintenance for eszopiclone tablets in a placebo-controlled 44-day study, and by subjective assessments of time to sleep onset and wake time after sleep onset (WASO) in a placebo-controlled study for 6 months. -
10 OVERDOSAGE
In clinical trials with eszopiclone, one case of overdose with up to 36 mg of eszopiclone was reported in which the subject fully recovered. Since commercial marketing began, spontaneous cases of eszopiclone overdoses up to 270 mg (90 times the maximum recommended dose of eszopiclone) have been reported, in which patients have recovered. Fatalities related to eszopiclone tablets overdoses were reported only in combination with other CNS drugs or alcohol.
10.1 Signs and Symptoms
Signs and symptoms of overdose effects of CNS depressants can be expected to present as exaggerations of the pharmacological effects noted in preclinical testing. Impairment of consciousness ranging from somnolence to coma has been described. Rare individual instances of fatal outcomes following overdose with racemic zopiclone have been reported in European postmarketing reports, most often associated with overdose with other CNS-depressant agents. Methemoglobinemia in association with overdoses of racemic zopiclone has been reported.
10.2 Recommended Treatment
General symptomatic and supportive measures should be used along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. Flumazenil may be useful. As in all cases of drug overdose, respiration, pulse, blood pressure, and other appropriate signs should be monitored and general supportive measures employed. Hypotension and CNS depression should be monitored and treated by appropriate medical intervention. Consider monitoring methemoglobin in the setting of high-dose overdosage. The value of dialysis in the treatment of overdosage has not been determined.
As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of hypnotic drug product overdosage. -
11 DESCRIPTION
Eszopiclone is a hypnotic agent/nonbenzodiazepine hypnotic agent that is a pyrrolopyrazine derivative of the cyclopyrrolone class. The chemical name of eszopiclone is (+)-4-Methyl-1-piperazinecarboxylic acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5 Hpyrrolo[3,4- b]pyrazin-5-(S)-yl ester. Its molecular weight is 388.81, and its molecular formula is C 17H 17ClN 6O 3. Eszopiclone has a single chiral center with an ( S)-configuration. It has the following chemical structure:
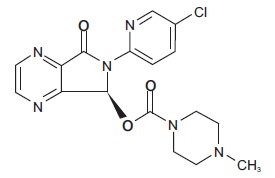
Eszopiclone USP is a white to slight yellowish powder. Eszopiclone USP is soluble in methylene chloride and dilute hydrochloric acid; practically insoluble in water and in alcohol.
Eszopiclone is formulated as film-coated tablets for oral administration. Eszopiclone tablets, USP contain 1 mg, 2 mg, or 3 mg eszopiclone USP and the following inactive ingredients: anhydrous dibasic calcium phosphate, croscarmellose sodium, colloidal silicon dioxide, hypromellose lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and triacetin. In addition, both the 1 mg and 3 mg tablets contain FD&C Blue #2/indigo carmine aluminium lake. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of eszopiclone as a hypnotic is unclear; however, its effect could be related to its interaction with GABA-receptor complexes at binding domains located close to or allosterically coupled to benzodiazepine receptors.
12.3 Pharmacokinetics
The pharmacokinetics of eszopiclone have been investigated in healthy subjects (adult and elderly) and in patients with hepatic disease or renal disease. In healthy subjects, the pharmacokinetic profile was examined after single doses of up to 7.5 mg and after once-daily administration of 1, 3, and 6 mg for 7 days. Eszopiclone is rapidly absorbed, with a time to peak concentration (t max)of approximately 1 hour and a terminal-phase elimination half-life (t 1/2) of approximately 6 hours. In healthy adults, eszopiclone tablets does not accumulate with once-daily administration, and its exposure is dose-proportional over the range of 1 to 6 mg.
Absorption and Distribution
Eszopiclone is rapidly absorbed following oral administration. Peak plasma concentrations are achieved within approximately 1 hour after oral administration. Eszopiclone is weakly bound to plasma protein (52 to 59%). The large free fraction suggests that eszopiclone disposition should not be affected by drug-drug interactions caused by protein binding. The blood-to-plasma ratio for eszopiclone is less than one, indicating no selective uptake by red blood cells.
Metabolism
Following oral administration, eszopiclone is extensively metabolized by oxidation and demethylation. The primary plasma metabolites are ( S)-zopiclone-N-oxide and ( S)-N-desmethyl zopiclone; the latter compound binds to GABA receptors with substantially lower potency than eszopiclone, and the former compound shows no significant binding to this receptor. In vitrostudies have shown that CYP3A4 and CYP2E1 enzymes are involved in the metabolism of eszopiclone. Eszopiclone did not show any inhibitory potential on CYP450 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 in cryopreserved human hepatocytes.
Elimination
After oral administration, eszopiclone is eliminated with a mean t 1/2of approximately 6 hours. Up to 75% of an oral dose of racemic zopiclone is excreted in the urine, primarily as metabolites. A similar excretion profile would be expected for eszopiclone, the S-isomer of racemic zopiclone. Less than 10% of the orally administered eszopiclone dose is excreted in the urine as parent drug.
Effect of Food
In healthy adults, administration of a 3 mg dose of eszopiclone after a high-fat meal resulted in no change in AUC, a reduction in mean C maxof 21%, and delayed t maxby approximately 1 hour.
The half-life remained unchanged, approximately 6 hours. The effects of eszopiclone tablets on sleep onset may be reduced if it is taken with or immediately after a high-fat/heavy meal.
Specific Populations
Age
Compared with nonelderly adults, subjects 65 years and older had an increase of 41% in total exposure (AUC) and a slightly prolonged elimination of eszopiclone (t 1/2approximately 9 hours). C maxwas unchanged. Therefore, in elderly patients the dose should not exceed 2 mg.
Gender
The pharmacokinetics of eszopiclone in men and women are similar.
Race
In an analysis of data on all subjects participating in Phase 1 studies of eszopiclone, the pharmacokinetics for all races studied appeared similar.
Hepatic Impairment
Pharmacokinetics of a 2 mg eszopiclone dose were assessed in 16 healthy volunteers and in 8 subjects with mild, moderate, and severe liver disease. Exposure was increased 2-fold in severely impaired patients compared with the healthy volunteers. C maxand t maxwere unchanged. No dose adjustment is necessary for patients with mild-to-moderate hepatic impairment. Dose reduction is recommended for patients with severe hepatic impairment. Eszopiclone tablets should be used with caution in patients with hepatic impairment [see Dosage and Administration (2.3)].
Renal Impairment
The pharmacokinetics of eszopiclone were studied in 24 patients with mild, moderate, or severe renal impairment. AUC and C maxwere similar in the patients compared with demographically matched healthy control subjects. No dose adjustment is necessary in patients with renal impairment, since less than 10% of the orally administered eszopiclone dose is excreted in the urine as parent drug.
Drug Interactions
Eszopiclone is metabolized by CYP3A4 and CYP2E1 via demethylation and oxidation. There were no pharmacokinetic or pharmacodynamic interactions between eszopiclone and paroxetine. When eszopiclone was coadministered with olanzapine, no pharmacokinetic interaction was detected in levels of eszopiclone or olanzapine, but a pharmacodynamic interaction was seen on a measure of psychomotor function. Eszopiclone and lorazepam decreased each other’s C maxby 22%. Coadministration of eszopiclone 3 mg to subjects receiving ketoconazole, a potent inhibitor of CYP3A4, 400 mg daily for 5 days, resulted in a 2.2-fold increase in exposure to eszopiclone. C maxand t 1/2were increased 1.4-fold and 1.3-fold, respectively. Eszopiclone tablets would not be expected to alter the clearance of drugs metabolized by common CYP450 enzymes [see Warnings and Precautions (5.7), Dosage and Administration (2.3)].
Paroxetine:Coadministration of single dose of eszopiclone and paroxetine produced no pharmacokinetic or pharmacodynamic interaction. The lack of a drug interaction following single-dose administration does not predict the complete absence of a pharmacodynamic effect following chronic administration.
Lorazepam:Coadministration of single doses of eszopiclone and lorazepam did not have clinically relevant effects on the pharmacodynamics or pharmacokinetics of either drug. The lack of a drug interaction following single-dose administration does not predict the complete absence of a pharmacodynamic effect following chronic administration.
Drugs with a Narrow Therapeutic Index
Digoxin:A single dose of eszopiclone 3 mg did not affect the pharmacokinetics of digoxin measured at steady state following dosing of 0.5 mg twice daily for one day and 0.25 mg daily for the next 6 days.
Warfarin:Eszopiclone 3 mg administered daily for 5 days did not affect the pharmacokinetics of ( R)- or ( S)-warfarin, nor were there any changes in the pharmacodynamic profile (prothrombin time) following a single 25 mg oral dose of warfarin.
Drugs Highly Bound to Plasma Protein
Eszopiclone is not highly bound to plasma proteins (52 to 59% bound); therefore, the disposition of eszopiclone is not expected to be sensitive to alterations in protein binding. Administration of eszopiclone 3 mg to a patient taking another drug that is highly protein-bound would not be expected to cause an alteration in the free concentration of either drug. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Carcinogenesis
In a carcinogenicity study in rats, oral administration of eszopiclone for 97 (males) or 104 (females) weeks resulted in no increases in tumors; plasma levels (AUC) of eszopiclone at the highest dose tested (16 mg/kg/day) are approximately 80 (females) and 20 (males) times those in humans at the MRHD of 3 mg/day. However, in a 2-year carcinogenicity study in rats, oral administration of racemic zopiclone (1, 10, or 100 mg/kg/day) resulted in increases in mammary gland adenocarcinomas (females) and thyroid gland follicular cell adenomas and carcinomas (males) at the highest dose tested. Plasma levels of eszopiclone at this dose are approximately 150 (females) and 70 (males) times those in humans at the MRHD of eszopiclone. The mechanism for the increase in mammary adenocarcinomas is unknown. The increase in thyroid tumors is thought to be due to increased levels of TSH secondary to increased metabolism of circulating thyroid hormones, a mechanism not considered relevant to humans.
In a 2-year carcinogenicity study in mice, oral administration of racemic zopiclone (1, 10, or 100 mg/kg/day) produced increases in pulmonary carcinomas and carcinomas plus adenomas (females) and skin fibromas and sarcomas (males) at the highest dose tested. The skin tumors were due to skin lesions induced by aggressive behavior, a mechanism not relevant to humans. A carcinogenicity study of eszopiclone was conducted in mice at oral doses up to 100 mg/kg/day. Although this study did not reach a maximum tolerated dose, and was thus inadequate for overall assessment of carcinogenic potential, no increases in either pulmonary or skin tumors were seen at doses producing plasma levels of eszopiclone approximately 90 times those in humans at the MRHD of eszopiclone (and 12 times the exposure in the racemate study).
Eszopiclone did not increase tumors in a p53 transgenic mouse bioassay at oral doses up to 300 mg/kg/day.
Mutagenesis
Eszopiclone was clastogenic in in vitro(mouse lymphoma and chromosomal aberration) assays in mammalian cells. Eszopiclone was negative in the in vitrobacterial gene mutation (Ames) assay and in an in vivomicronucleus assay.
( S)-N-desmethyl zopiclone, a metabolite of eszopiclone, was positive in in vitrochromosomal aberration assays in mammalian cells. ( S)-N-desmethyl zopiclone was negative in the in vitrobacterial gene mutation (Ames) assay and in an in vivochromosomal aberration and micronucleus assay.
Impairment of Fertility
Oral administration of eszopiclone to rats prior to and during mating, and continuing in females to day 7 of gestation (doses up to 45 mg/kg/day to males and females or up to 180 mg/kg/day to females only) resulted in decreased fertility, with no pregnancy at the highest dose tested when both males and females were treated. In females, there was an increase in abnormal estrus cycles at the highest dose tested. In males, decreases in sperm number and motility and increases in morphologically abnormal sperm were observed at the mid and high doses. The no-effect dose for adverse effects on fertility (5 mg/kg/day) is 16 times the MRHD on a mg/m 2basis. -
14 CLINICAL STUDIES
The effect of eszopiclone tablets on reducing sleep latency and improving sleep maintenance was established in studies with 2100 subjects (ages 18 to 86) with chronic and transient insomnia in six placebo-controlled trials of up to 6 months’ duration. Two of these trials were in elderly patients (n=523). Overall, at the recommended adult dose (2 to 3 mg) and elderly dose (1 to 2 mg), eszopiclone tablets significantly decreased sleep latency and improved measures of sleep maintenance (objectively measured as wake time after sleep onset [WASO] and subjectively measured as total sleep time).
14.1 Transient Insomnia
Healthy adults were evaluated in a model of transient insomnia (n=436) in a sleep laboratory in a double-blind, parallel-group, single-night trial comparing two doses of eszopiclone and placebo. Eszopiclone tablets 3 mg was superior to placebo on measures of sleep latency and sleep maintenance, including polysomnographic (PSG) parameters of latency to persistent sleep (LPS) and WASO.
14.2 Chronic Insomnia (Adults and Elderly)
The effectiveness of eszopiclone tablets was established in five controlled studies in chronic insomnia. Three controlled studies were in adult subjects, and two controlled studies were in elderly subjects with chronic insomnia.
Adults
In the first study, adults with chronic insomnia (n=308) were evaluated in a double-blind, parallel-group trial of 6 weeks’ duration comparing eszopiclone tablets 2 mg and 3 mg with placebo. Objective endpoints were measured for 4 weeks. Both 2 mg and 3 mg were superior to placebo on LPS at 4 weeks. The 3 mg dose was superior to placebo on WASO.
In the second study, adults with chronic insomnia (n=788) were evaluated using subjective measures in a double-blind, parallel-group trial comparing the safety and efficacy of eszopiclone tablets 3 mg with placebo administered nightly for 6 months. Eszopiclone tablets was superior to placebo on subjective measures of sleep latency, total sleep time, and WASO.
In addition, a 6-period crossover PSG study evaluating eszopiclone doses of 1 to 3 mg, each given over a 2-day period, demonstrated effectiveness of all doses on LPS, and of 3 mg on WASO. In this trial, the response was dose related.
Elderly
Elderly subjects (ages 65 to 86 years) with chronic insomnia were evaluated in two double-blind, parallel-group trials of 2 weeks duration. One study (n=231) compared the effects of eszopiclone tablets with placebo on subjective outcome measures, and the other (n=292) on objective and subjective outcome measures. The first study compared 1 mg and 2 mg of eszopiclone tablets with placebo, while the second study compared 2 mg of eszopiclone tablets with placebo. All doses were superior to placebo on measures of sleep latency. In both studies, 2 mg of eszopiclone tablets was superior to placebo on measures of sleep maintenance.14.3 Studies Pertinent to Safety Concerns for Sedative Hypnotic Drugs
Next-Day Residual Effects
In a double-blind study of 91 healthy adults age 25-to 40 years, the effects of eszopiclone tablets 3 mg on psychomotor function were assessed between 7.5 and 11.5 hours the morning after dosing. Measures included tests of psychomotor coordination that are correlated with ability to maintain a motor vehicle in the driving lane, tests of working memory, and subjective perception of sedation and coordination. Compared with placebo, eszopiclone tablets 3 mg was associated with next-morning psychomotor and memory impairment that was most severe at 7.5 hours, but still present and potentially clinically meaningful at 11.5 hours. Subjective perception of sedation and coordination from eszopiclone tablets 3 mg was not consistently different from placebo, even though subjects were objectively impaired.
In a 6-month double-blind, placebo-controlled trial of nightly administered eszopiclone tablets 3 mg, memory impairment was reported by 1.3% (8/593) of subjects treated with eszopiclone tablets 3 mg compared to 0% (0/195) of subjects treated with placebo. In a 6-week adult study of nightly administered eszopiclone tablets confusion was reported by 3.0% of patients treated with eszopiclone tablets 3 mg, compared to 0% of subjects treated with placebo. In the same study, memory impairment was reported by 1% of patients treated with either 2 mg or 3 mg eszopiclone tablets, compared to 0% treated with placebo.
In a 2-week study of 264 elderly insomniacs, 1.5% of patients treated with eszopiclone tablets 2 mg reported memory impairment compared to 0% treated with placebo. In another 2-week study of 231 elderly insomniacs, 2.5% of patients treated with eszopiclone tablets 2 mg reported confusion compared to 0% treated with placebo.
Withdrawal-Emergent Anxiety and Insomnia
During nightly use for an extended period, pharmacodynamic tolerance or adaptation has been observed with other hypnotics. If a drug has a short elimination half-life, it is possible that a relative deficiency of the drug or its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night’s use. This is believed to be responsible for two clinical findings reported to occur after several weeks of nightly use of other rapidly eliminated hypnotics: increased wakefulness during the last quarter of the night and the appearance of increased signs of daytime anxiety.
In a 6-month double-blind, placebo-controlled study of nightly administration of eszopiclone tablets 3 mg, rates of anxiety reported as an adverse event were 2.1% in the placebo arm and 3.7% in the eszopiclone tablets arm. In a 6-week adult study of nightly administration, anxiety was reported as an adverse event in 0%, 2.9%, and 1.0% of the placebo, 2 mg, and 3 mg treatment arms, respectively. In this study, single-blind placebo was administered on nights 45 and 46, the first and second days of withdrawal from study drug. New adverse events were recorded during the withdrawal period, beginning with day 45, up to 14 days after discontinuation. During this withdrawal period, 105 subjects previously taking nightly eszopiclone tablets 3 mg for 44 nights spontaneously reported anxiety (1%), abnormal dreams (1.9%), hyperesthesia (1%), and neurosis (1%), while none of 99 subjects previously taking placebo reported any of these adverse events during the withdrawal period.
Rebound insomnia, defined as a dose-dependent temporary worsening in sleep parameters (latency, sleep efficiency, and number of awakenings) compared with baseline following discontinuation of treatment, is observed with short- and intermediate-acting hypnotics. Rebound insomnia following discontinuation of eszopiclone tablets relative to placebo and baseline was examined objectively in a 6-week adult study on the first 2 nights of discontinuation (nights 45 and 46) following 44 nights of active treatment with 2 mg or 3 mg. In the eszopiclone tablets 2 mg group, compared with baseline, there was a significant increase in WASO and a decrease in sleep efficiency, both occurring only on the first night after discontinuation of treatment. No changes from baseline were noted in the eszopiclone tablets 3 mg group on the first night after discontinuation, and there was a significant improvement in LPS and sleep efficiency compared with baseline following the second night of discontinuation. Comparisons of changes from baseline between eszopiclone tablets and placebo were also performed. On the first night after discontinuation of eszopiclone tablets 2 mg, LPS and WASO were significantly increased and sleep efficiency was reduced; there were no significant differences on the second night. On the first night following discontinuation of eszopiclone tablets 3 mg, sleep efficiency was significantly reduced. No other differences from placebo were noted in any other sleep parameter on either the first or second night following discontinuation. For both doses, the discontinuation-emergent effect was mild, had the characteristics of the return of the symptoms of chronic insomnia, and appeared to resolve by the second night after eszopiclone tablets discontinuation. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Eszopiclone tablets USP, 3 mg are dark blue to blue, round, biconvex, film-coated tablets debossed with ‘H’ on one side and ‘E16’ on the other side.
Bottles of 30 tablets NDC: 31722-857-30
Bottles of 100 tablets NDC: 31722-857-01
Eszopiclone tablets USP, 2 mg are white to off-white, round, biconvex, film-coated tablets debossed with ‘H’ on one side and ‘E15’ on the other side.
Bottles of 30 tablets NDC: 31722-856-30
Bottles of 100 tablets NDC: 31722-856-01
Eszopiclone tablets USP, 1 mg are light blue, round, biconvex, film-coated tablets debossed with ‘H’ on one side and ‘E14’ on the other side.
Bottles of 30 tablets NDC: 31722-855-30
Bottles of 100 tablets NDC: 31722-855-01
Store at 20° to 25° C (68° to 77° F) [see USP Controlled Room Temperature]. -
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling ( Medication Guide).
Inform patients and their families about the benefits and risks of treatment with eszopiclone tablets. Inform patients of the availability of a Medication Guide and instruct them to read the Medication Guide prior to initiating treatment with eszopiclone tablets and with each prescription refill. Review the eszopiclone tablets Medication Guide with every patient prior to initiation of treatment. Instruct patients or caregivers that eszopiclone tablets should be taken only as prescribed.
Complex Sleep Behaviors
Instruct patients and their families that ESZOPICLONE TABLETS may cause complex sleep behaviors, including sleep-walking, sleep-driving, preparing and eating food, making phone calls, or having sex while not being fully awake. Serious injuries and death have occurred during complex sleep behavior episodes. Tell patients to discontinue ESZOPICLONE TABLETS and notify their healthcare provider immediately if they develop any of these symptoms [see Boxed Warning, Warnings and Precautions (5.1)].
CNS Depressant Effects and Next-Day Impairment
Tell patients that eszopiclone tablets can cause next-day impairment even when used as prescribed, and that this risk is increased if dosing instructions are not carefully followed. Caution patients taking the 3 mg dose against driving and other activities requiring complete mental alertness the day after use. Inform patients that impairment can be present despite feeling fully awake. Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients [see Warnings and Precautions (5.2)].
Severe Anaphylactic and Anaphylactoid Reactions
Inform patients that severe anaphylactic and anaphylactoid reactions have occurred with eszopiclone. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if any of them occur [see Warnings and Precautions (5.4)].
Suicide
Tell patients to immediately report any suicidal thoughts.
Alcohol and Other Drugs
Ask patients about alcohol consumption, medicines they are taking, and drugs they may be taking without a prescription. Advise patients not to use eszopiclone tablets if they drank alcohol that evening or before bed.
Tolerance, Abuse, and Dependence
Tell patients not to increase the dose of eszopiclone tablets on their own, and to inform you if they believe the drug "does not work.”
Administration Instructions
Patients should be counseled to take eszopiclone tablets right before they get into bed and only when they are able to stay in bed a full night (7 to 8 hours) before being active again. Eszopiclone tablets should not be taken with or immediately after a meal. Advise patients NOT to take eszopiclone tablets if they drank alcohol that evening.
Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By: Annora Pharma Pvt. Ltd.
Sangareddy - 502313, Telangana, India.
Revised: 01/2023 -
MEDICATION GUIDE
Eszopiclone Tablets, USP C-IV
(ess-ZOP-ih-klone)
Read the Medication Guide that comes with eszopiclone tablets before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about your medical condition or treatment.
What is the most important information I should know about eszopiclone tablets?
Do not take more eszopiclone tablets than prescribed.
Do not take eszopiclone tablets unless you are able to stay in bed a full night (7 to 8 hours) before you must be active again.
Take eszopiclone tablets right before you get in bed, not sooner.
Eszopiclone tablets may cause serious side effects, including:
Complex sleep behaviors that have caused serious injury and death.After taking eszopiclone tablets, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing (complex sleep behaviors). The next morning, you may not remember that you did anything during the night. These activities may occur with eszopiclone tablets whether or not you drink alcohol or take other medicines that make you sleepy.
Reported activities and behaviors include:
doing activities when you are asleep like:
o making and eating food
o talking on the phone
o having sex
o driving a car (“sleep-driving”)
o sleep walking
Stop taking eszopiclone tablets and call your healthcare provider right away if you find out that you have done any of the above activities after taking eszopiclone tablets.
The morning after you take eszopiclone tablets your ability to drive safely and think clearly may be decreased. You also may experience sleepiness during the day.
Do not take eszopiclone tablets if you:
have ever experienced a complex sleep behavior (such as driving a car, making and eating food, talking on the phone or having sex while not fully awake) after taking eszopiclone tablets.
drank alcohol that evening or before bed
take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take eszopiclone tablets with your other medicines.
cannot get a full night’s sleep
WHAT ARE ESZOPICLONE TABLETS?
Eszopiclone tablets are a sedative-hypnotic (sleep) medicine. Eszopiclone tablets are used in adults for the treatment of a sleep problem called insomnia. Symptoms of insomnia include:
trouble falling asleep
waking up often during the night
Eszopiclone tablets are not for children.
Eszopiclone tablets are a federally controlled substance (C-IV) because it can be abused or lead to dependence. Keep eszopiclone tablets in a safe place to prevent misuse and abuse. Selling or giving away eszopiclone tablets may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs. Who should not take eszopiclone tablets?
Do not take eszopiclone tablets if you have ever had a complex sleep behavior happen after taking eszopiclone tablets.
Do not take eszopiclone tablets if you are allergic to anything in it. See the end of this Medication Guide for a complete list of ingredients in eszopiclone tablets.
Eszopiclone tablets may not be right for you. Before starting eszopiclone tablets, tell your doctor about all of your health conditions, including if you:
have a history of depression, mental illness, or suicidal thoughts
have a history of drug or alcohol abuse or addiction
have liver disease
are pregnant, planning to become pregnant, or breastfeeding
Tell your doctor about all of the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Medicines can interact with each other, sometimes causing serious side effects. Do not take eszopiclone tablets with other medicines that can make you sleepy.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How should I take eszopiclone tablets?
Take eszopiclone tablets exactly as prescribed. Do not take more eszopiclone tablets than prescribed for you.
Take eszopiclone tablets right before you get into bed.
Do not take eszopiclone tablets with or right after a meal.
Do not take eszopiclone tablets unless you are able to get a full night’s sleep before you must be active again.
Call your doctor if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problems.
If you take too much eszopiclone tablets or overdose, call your doctor or poison control center right away, or get emergency treatment.
What are the possible side effects of eszopiclone tablets?
Possible serious side effects of eszopiclone tablets include:
getting out of bed while not being fully awake and doing an activity that you do not know you are doing.(See “What is the most important information I should know about eszopiclone tablets?”)
abnormal thoughts and behavior.Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, acting strangely, hallucinations, worsening of depression, and suicidal thoughts or actions.
memory loss
anxiety
severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing, and nausea and vomiting. Get emergency medical help if you get these symptoms after taking eszopiclone tablets.
Call your doctor right away if you have any of the above side effects or any other side effects that worry you while using eszopiclone tablets.
The most common side effects of eszopiclone tablets are:
unpleasant taste in mouth, dry mouth
drowsiness
dizziness
headache
symptoms of the common cold
You may still feel drowsy the next day after taking eszopiclone tablets. Do not drive or do other dangerous activities after taking eszopiclone tablets until you feel fully awake.
These are not all the side effects of eszopiclone tablets. Ask your doctor or pharmacist for more information. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store eszopiclone tablets?
Store eszopiclone tablets at 68°F to 77° F (20°C to 25° C) .
Do not use eszopiclone tablets after the expiration date.
Keep eszopiclone tablets and all medicines out of reach of children.
General Information about eszopiclone tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
Do not use eszopiclone tablets for a condition for which it was not prescribed.
Do not share eszopiclone tablets with other people, even if you think they have the same symptoms that you have. It may harm them and is against the law.
This Medication Guide summarizes the most important information about eszopiclone tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about eszopiclone tablets that is written for healthcare professionals.
For more information, call 1-866-495-1995.
What are the ingredients in eszopiclone tablets?
Active Ingredient: eszopiclone USP
Inactive Ingredients:calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and triacetin. In addition, both the 1 mg and 3 mg tablets contain FD&C Blue #2/indigo carmine aluminium lake.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Medication Guide available at http://camberpharma.com/medication-guides

Manufactured for:
Camber Pharmaceuticals, Inc.
Piscataway, NJ 08854
By: Annora Pharma Pvt. Ltd.
Sangareddy - 502313, Telangana, India.
Revised: 01/2023
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ESZOPICLONE
eszopiclone tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 31722-855 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESZOPICLONE (UNII: UZX80K71OE) (ESZOPICLONE - UNII:UZX80K71OE) ESZOPICLONE 1 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue (Light Blue) Score no score Shape ROUND Size 6mm Flavor Imprint Code H;E14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 31722-855-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2024 2 NDC: 31722-855-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205504 01/04/2024 ESZOPICLONE
eszopiclone tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 31722-856 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESZOPICLONE (UNII: UZX80K71OE) (ESZOPICLONE - UNII:UZX80K71OE) ESZOPICLONE 2 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (white to off-white) Score no score Shape ROUND Size 6mm Flavor Imprint Code H;E15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 31722-856-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2024 2 NDC: 31722-856-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205504 01/04/2024 ESZOPICLONE
eszopiclone tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 31722-857 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESZOPICLONE (UNII: UZX80K71OE) (ESZOPICLONE - UNII:UZX80K71OE) ESZOPICLONE 3 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue (dark blue to blue) Score no score Shape ROUND Size 6mm Flavor Imprint Code H;E16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 31722-857-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2024 2 NDC: 31722-857-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205504 01/04/2024 Labeler - Camber Pharmaceuticals, Inc. (826774775) Establishment Name Address ID/FEI Business Operations Annora Pharma Private Limited 650980746 analysis(31722-855, 31722-856, 31722-857) , manufacture(31722-855, 31722-856, 31722-857)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.