SODIUM FLUORIDE solution/ drops
Sodium Fluoride by
Drug Labeling and Warnings
Sodium Fluoride by is a Other medication manufactured, distributed, or labeled by Sancilio & Company Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- HEALTH CLAIM
-
Description
Each mL of Sodium Fluoride Drops contains 0.5 mg Fluoride ion (F) from 1.1 mg Sodium Fluoride (NaF). For use as a dental caries preventive in pediatric patients. No dyes, artificial flavors or sugar. Saccharin free. Gluten free.
Active Ingredients: Sodium Fluoride (0.11% w/v).
Other Ingredients: Glycerin, purified water, xylitol, propylene glycol, natural grape flavor, sucralose, methyl paraben, propyl paraben.
FLUORIDE SUPPLEMENT DOSAGE SCHEDULE§ AGE Fluoride Ion Level in Drinking Water (ppm)* < 0.3 ppm 0.3 - 0.6 ppm > 0.6 ppm - * 1.0 ppm = 1 mg/Liter
- † 1.1 mg Sodium Fluoride contains 0.5 mg Fluoride ion
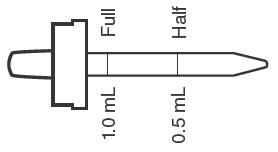
Birth to 6 months None None None 6 months to 3 years Half dropperful
0.25 mg F (1/2 mL)None None 3 to 6 years One dropperful
0.5 mg F (1 mL)†Half dropperful
0.25 mg F (1/2 mL)None 6 to 16 years Two dropperfuls
1 mg F (2 mL)One dropperful
0.5 mg F (1 mL)None Fluoride Supplement Dose Schedule approved by the American Dental Association, American Academy of Pediatrics and American Academy of Pediatric Dentistry.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
- Clinical Pharmacology
-
Indications and Usage
As a supplemental source of Fluoride. It has been established that ingestion of fluoridated drinking water (1 ppm F) during the period of tooth development results in significant decrease in the incidence of dental caries.1 Sodium Fluoride Drops were developed to provide systemic Fluoride for use as a supplement in pediatric patients from 6 months to age 3 and older, living in areas where the drinking water Fluoride level does not exceed 0.6 ppm F.
- Contraindications
-
Warnings
Prolonged daily ingestion of quantities greater than the recommended amount may result in various degrees of dental fluorosis in pediatric patients under age 6 years, especially if the water fluoridation exceeds 0.6 ppm. Read directions carefully before using. Keep out of the reach of infants and children.
-
Precautions
See "Overdosage" section. Incompatibility of Fluoride with dairy foods has been reported due to formation of Calcium Fluoride which is poorly absorbed. Not for use in the eyes.
- Adverse Reactions
- HEALTH CLAIM
- TAMPER EVIDENT
-
Overdosage
Prolonged daily ingestion of excessive Fluoride may result in varying degrees of dental fluorosis. The total amount of Sodium Fluoride in a bottle of 50 mL (0.5 mg/mL) Sodium Fluoride Drops (25 mg F) conforms with the recommendations of the American Dental Association for the maximum to be dispensed at one time for safety purposes. If overdose is suspected, call 1-800-222-1222 (American Association of Poison Control Centers), your local poison control center (www.aapcc.org), or emergency room immediately for treatment recommendations.
-
DOSAGE & ADMINISTRATION
Dosage§ and Administration
Daily oral dose: (in areas where the drinking water contains less than 0.3 ppm F) age 6 months to 3 years: one half dropperful (1/2 mL); age 3 to 6 years, one dropperful (1 mL); age 6 to 16 years, two dropperfuls (2 mL). When drinking water is partially fluoridated (0.3 to 0.6 ppm F inclusive) dose as follows: age 6 months to 3 years, Fluoride supplementation not indicated; age 3 to 6 years, one half dropperful (1/2 mL); age 6 to 16 years, one dropperful (1 mL).
- How Supplied
- References
- HEALTH CLAIM
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 50 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
SODIUM FLUORIDE
sodium fluoride solution/ dropsProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:44946-1032 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Xylitol (UNII: VCQ006KQ1E) Propylene glycol (UNII: 6DC9Q167V3) Sucralose (UNII: 96K6UQ3ZD4) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:44946-1032-8 50 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Dietary Supplement 12/20/2011 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value flavor Labeler - Sancilio & Company Inc (176681257)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.