Pigment by Physicians Care Alliance, LLC (d/b/a PCA Skin) Pigment
Pigment by
Drug Labeling and Warnings
Pigment by is a Otc medication manufactured, distributed, or labeled by Physicians Care Alliance, LLC (d/b/a PCA Skin). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
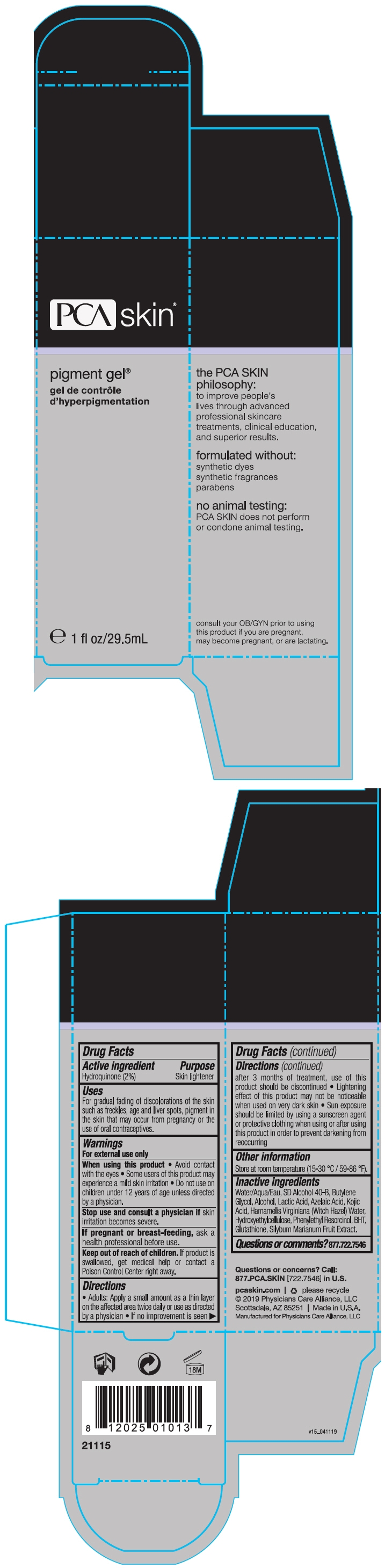
PIGMENT- hydroquinone gel
Physicians Care Alliance, LLC (d/b/a PCA Skin)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Pigment
Uses
For gradual fading of discolorations of the skin such as freckles, age and liver spots, pigment in the skin that may occur from pregnancy or the use of oral contraceptives.
Warnings
For external use only
Directions
- Adults: Apply a small amount as a thin layer on the affected area twice daily or use as directed by a physician
- If no improvement is seen after 3 months of treatment, use of this product should be discontinued
- Lightening effect of this product may not be noticeable when used on very dark skin
- Sun exposure should be limited by using a sunscreen agent or protective clothing when using or after using this product in order to prevent darkening from reoccurring
| PIGMENT
hydroquinone gel |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Physicians Care Alliance, LLC (d/b/a PCA Skin) (969945133) |
Revised: 8/2020
Document Id: cb295c18-9fb3-4321-80b4-0982ced6e131
Set id: 014f337e-d0b1-4505-9b84-0a777913692f
Version: 1
Effective Time: 20200812
Physic
Trademark Results [Pigment]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PIGMENT 87790871 5561054 Live/Registered |
Copper Frog Games LLC 2018-02-08 |
 PIGMENT 86867729 5017939 Live/Registered |
PIXITE INC. 2016-01-06 |
 PIGMENT 85545195 not registered Dead/Abandoned |
Kaura, Samantha H 2012-02-16 |
 PIGMENT 79373301 not registered Live/Pending |
PIGMENT 2023-04-07 |
 PIGMENT 79223795 5651925 Live/Registered |
Pigment Productions Limited 2017-10-13 |
 PIGMENT 78218587 not registered Dead/Abandoned |
Meyers, Chuck 2003-02-25 |
 PIGMENT 74079803 not registered Dead/Abandoned |
Wasko, Carl, Jr. 1990-07-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.