GEN7T PLUS- lidocaine, menthol patch
Gen7T Plus by
Drug Labeling and Warnings
Gen7T Plus by is a Prescription medication manufactured, distributed, or labeled by Beijing HKKY Medical Tech. Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
Gen7T Plus Patch
---------
What is Gen7T Plus Patch®?
This is a topical hydrogel patch consisting of the local anesthetic, lidocaine, and the topical analgesic, menthol.
What is Gen7T PlusPatch®used for?
This patch is applied topically for use on normal intact skin for the temporary relief of minor aches and pains of muscles and joints, such as simple backache, lumbago, arthritis, neuralgia, strains, bruises, and sprains. Read the information sheet provided before you start using this medication and each time you get a refill. If you have any questions, please consult your doctor or pharmacist. Inform your doctor if your condition does not improve or if it worsens. The use of this medication shall be used under the supervision of a physician only as it may be used in conjunction with other therapies.
This information may not include all of the information needed to use Gen7T Plus Patch ® safely and effectively.
For Topical Use Only
What are the possible side effects with Gen7T PlusPatch®
- Common: itching, redness or flaking of the skin following application (note: the majority of patients experience no significant adverse events following patch application).
- NOTE: serious side effects are, in general, related to accidental toxicity of medication by applying considerably more than directed by your doctor or pharmacist or by ingesting the contents of the patch or using this patch in conjunction with other lidocaine containing products.
- Tell your healthcare provider about all the medicines you take. This includes prescription and nonprescription medicines, vitamins, and herbal supplements.
- Avoid excessive alcohol usage, since it may increase the potential for Central Nervous System (CNS) effects such as dizziness, confusion, lightheadedness and orthostatic hypotension.
This is not a complete list of the possible side effects. For more information, talk with your doctor or pharmacist. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS FOR USE
Adults and children 12 years and over:
Apply 1 patch to the affected area of intact skin. Gen7T Plus Patch ® should be removed after 12 hours of continuous use and remain off for at least 12 hours.
- Determine area of patch application. If the pain area is smaller than the patch, patches may be cut into smaller sizes with scissors. Safely discard the remaining unused pieces of cut patches where children and pets cannot reach.
- Remove the transparent release liner (clear plastic backing) before application of patches to the skin. Apply immediately after removal from the protective envelope.
- Apply 1 patch to the affected area so that the patch covers most of the painful area. Apply patch only once during each 24-hr period (12 hours on / 12 hours off).
- Remove patch if irritation occurs.
Children under 12 years of age:Consult a doctor
- Determine area of patch application. If the pain area is smaller than the patch, patches may be cut into smaller sizes with scissors. Safely discard the remaining unused pieces of cut patches where children and pets cannot reach.
-
WARNINGS
FOR EXTERNAL USE ONLY
1. Do not use:
- More than 1 patch on your body at a time or on irritated or swollen skin
- On wounds, damaged or infected skin
- On eyes, mouth, genitals, or other mucous membranes
- With a heating pad
2. Consult physician for children under 12 years of age
3. Stop and consult your prescriber
- If condition or pain worsens
- If you are allergic to any of the ingredients in this product
- If using concurrently with any other external pain-relieving products
- If you are pregnant, planning to become pregnant, or breastfeeding
- If symptoms persist for more than 7 days, or symptoms clear up and occur again within 3 days
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- Shortness of breath
- Swelling or numbness of the tongue or throat
- Severe headache or vomiting
- Dizziness or faintness
- Changes in vision or speech
Excessive dosage, or short interval between doses, can result in high plasma levels and serious adverse effects. Patients should be instructed to strictly adhere to the recommended dosage and administration guidelines set forth in this literature and on your prescription label. The management of serious adverse reactions may require the use of resuscitative equipment, oxygen or other resuscitative drugs.
General information about the safe and effective use Gen7T PlusPatch®
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use this product for another indication unless instructed and prescribed by a physician. Do not give this drug to anyone else, even if they have the same condition. This product is intended for use as prescribed by a physician.
How should I store Gen7T PlusPatch®
Store product at room temperature at 68°F to 77°F (20°C to 25°C). Keep away from heat or sunlight. Protect from excessive moisture. Safely discard product after expiration date posted on the product label. Discard patches away from small children or animals.
DO NOT use the product after the expiration date printed on the box.
What are the active ingredients in Gen7T PlusPatch® ?
The patch consists of 3.5% lidocaine and 7% menthol.
- More than 1 patch on your body at a time or on irritated or swollen skin
-
INACTIVE INGREDIENTS
Polyvinyl alcohol, non-crystallizing sorbitol solution, polyacrylic acid, glycerin, carboxymethylcellulose sodium, colloidal silicon dioxide, titanium dioxide, propylene glycol, tartaric acid, magnesium hydroxide, sodium polyacrylate, purified water.
PRESCRIBER INFORMATION
Gen7T Plus Patch®(Lidocaine 3.5% / Menthol 7%)
-
DESCRIPTION
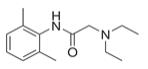
Gen7T PlusPatch® is a prescription topical patch containing 15 articulated patches or 10 articulated patches. Lidocaine is present in a 3.5% concentration (w/w). It is chemically designated as 2-(diethylamino)-N-(2,6-dimethylphenyl) acetamide and has an empirical formula of C 14H 22N 2O. The molecular weight of lidocaine is 234.34 g/mol. Lidocaine has an octanol: water partition ration of 43 at pH 7.4, and has the following structure:
** Lidocaine**
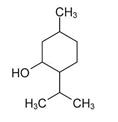
Menthol is present in a 7% concentration (w/w). The chemical name is (1R,2S,5R)‐2‐isopropyl-5-methylcyclohexanol. The empirical formula for Menthol is C 10H 20O with a molecular weight of 156.27 g/mol. Menthol contains colorless, hexangonal crystals, usually needle-like; fused masses or crystalline powder with a pleasant, peppermint-like odor. It has a melting point between 31 OC to 36 OC and has the following structure:
** Menthol**
Gen7T PlusPatch®uses an adhesive hydrogel technology containing lidocaine 3.5% and menthol 7%, applied to a flexible woven polyester backing and protected by a plastic film. The protective film is removed prior to application to the skin.
-
CLINICAL PHARMACOLOGY
Lidocaine is a topical anesthetic and stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses, thereby effecting local anesthetic action.
Menthol has local anesthetic and counterirritant qualities. It also acts as a weak kappa (ĸ) opioid receptor agonist. Menthol chemically triggers the cold-‐sensitive TRPM-8 receptors in the skin, which are responsible for the well-‐documented cooling sensation that occurs when applied to the skin. Menthol’s analgesic properties are not fully understood; however, they are mediated through a selective activation of ĸ-opioid receptors. Menthol also blocks voltage-‐sensitive sodium channels, reducing neural activity that may stimulate muscle tissue.
Menthol works by targeting the k-‐opioid receptor on the TRPM8 neuron. The TRPM8 neuron is normally activated at temperatures between (8°-28°C). Menthol causes the neuron to fire at temperatures above normal activation, which triggers the characteristic cooling sensation. Also because of menthol's specific targeting of the k-‐opioid receptor, it is endowed with analgesic properties.
Lidocaine is an amide-‐type local anesthetic agent and is suggested to stabilize neuronal membranes by inhibiting the ionic fluxes required for the initiation and conduction of impulses.
The penetration of Lidocaine into intact skin after application of patch is sufficient to produce an analgesic effect, but less than the amount necessary to produce a complete sensory block.
- CONTRAINDICATIONS
-
PRECAUTIONS
Because of the possibility of sedation, patients should be cautioned regarding the operation of heavy machinery or automobiles, and any activities made hazardous by decreased alertness.
Hepatic Disease: Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of Lidocaine, because of their inability to metabolize Lidocaine normally.
Allergic Reactions: Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to Lidocaine. However, this product should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain.
Non-intact Skin: Application to broken or inflamed skin, although not tested, may result in higher blood concentrations of Lidocaine from increased absorption. Gen7T PlusPatch®is only recommended for use on intact skin.
Eye Exposure: The contact of this product with the eyes, although not studied, should be avoided based on the findings of severe eye irritations with the use of similar products in animals. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
External Heat Sources: Placement of external heat sources, such as heating pads or electric blankets, over patches is not recommended as this has not been evaluated and may increase plasma Lidocaine levels.
DRUG INTERACTIONS
Antiarrhythmic Drugs:Gen7T PlusPatch®should be used with caution in patients receiving Class 1 antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic.
Local Anesthetics: When Gen7T PlusPatch®is used concurrently with other products containing local anesthetic agents the amount absorbed from all formulations must be considered.
CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY:
A minor metabolite, 2,6-xylidine, has been found to be carcinogenic in rats. The blood concentration of this metabolite is negligible following application of topical lidocaine. The effect of Gen7T PlusPatch®on fertility has not been studied.
PREGNANCY:
The safety of Gen7T PlusPatch® has not been established during pregnancy. There are no well-controlled studies in pregnant women. Gen7T PlusPatch®should not be used during pregnancy unless absolutely needed and discussed with a physician.
NURSING MOTHERS
The effect of Gen7T Plus Patch® on the nursing infant has not been evaluated. Caution should be exercised when Gen7T PlusPatch®is administered to a nursing mother.
PEDIATRIC / GERIATRIC USE
Safety and effectiveness in pediatric and geriatric patients have not been established.
ADVERSE REACTIONS
The most common adverse reactions are application site reactions, including dermatitis, itching or scaling. These tend to be dose-limiting and diminish with time.
Serious adverse experiences following the administration of Gen7T PlusPatch® are similar in nature to those observed in other amide anesthetic-containing agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage, rapid absorption, or may result from hypersensitivity, idiosyncrasy, or a diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature.
-
OVERDOSAGE
There have been no reports of over-dosage with Gen7T PlusPatch®. Signs of overdosage would include vomiting, drowsiness, coma, respiratory depression, and seizures. In the case of an overdosage, discontinue the product immediately, treat the patient symptomatically, and institute supportive measures.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GEN7T PLUS
lidocaine, menthol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71073-207 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 490 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 980 mg Inactive Ingredients Ingredient Name Strength POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TARTARIC ACID (UNII: W4888I119H) MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) WATER (UNII: 059QF0KO0R) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71073-207-10 10 in 1 BOX; Type 0: Not a Combination Product 07/29/2020 2 NDC: 71073-207-35 15 in 1 BOX; Type 0: Not a Combination Product 07/29/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/29/2020 Labeler - Beijing HKKY Medical Tech. Co., Ltd. (544434817) Establishment Name Address ID/FEI Business Operations Beijing HKKY Medical Tech. Co., Ltd. 544434817 manufacture(71073-207)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

