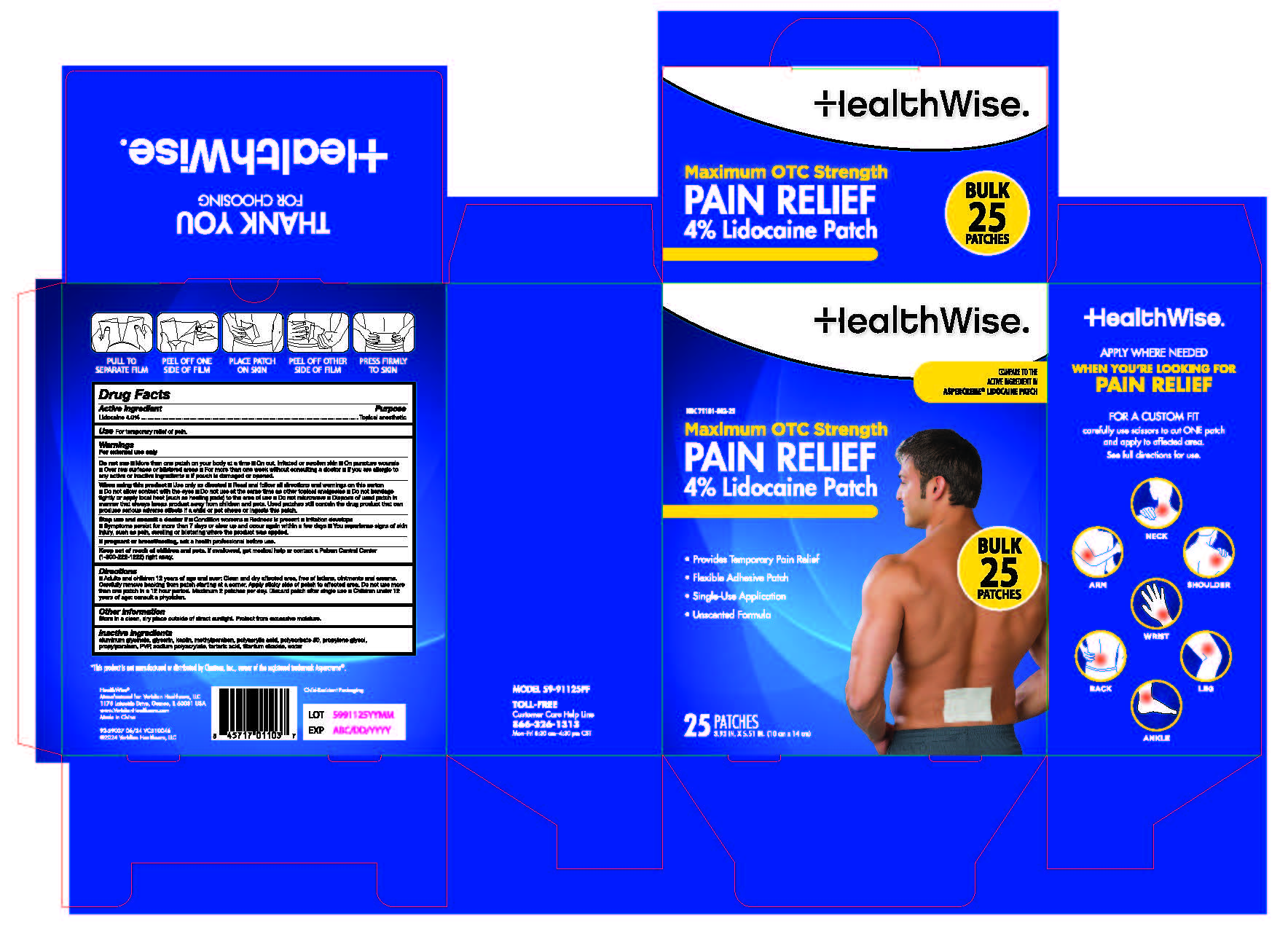
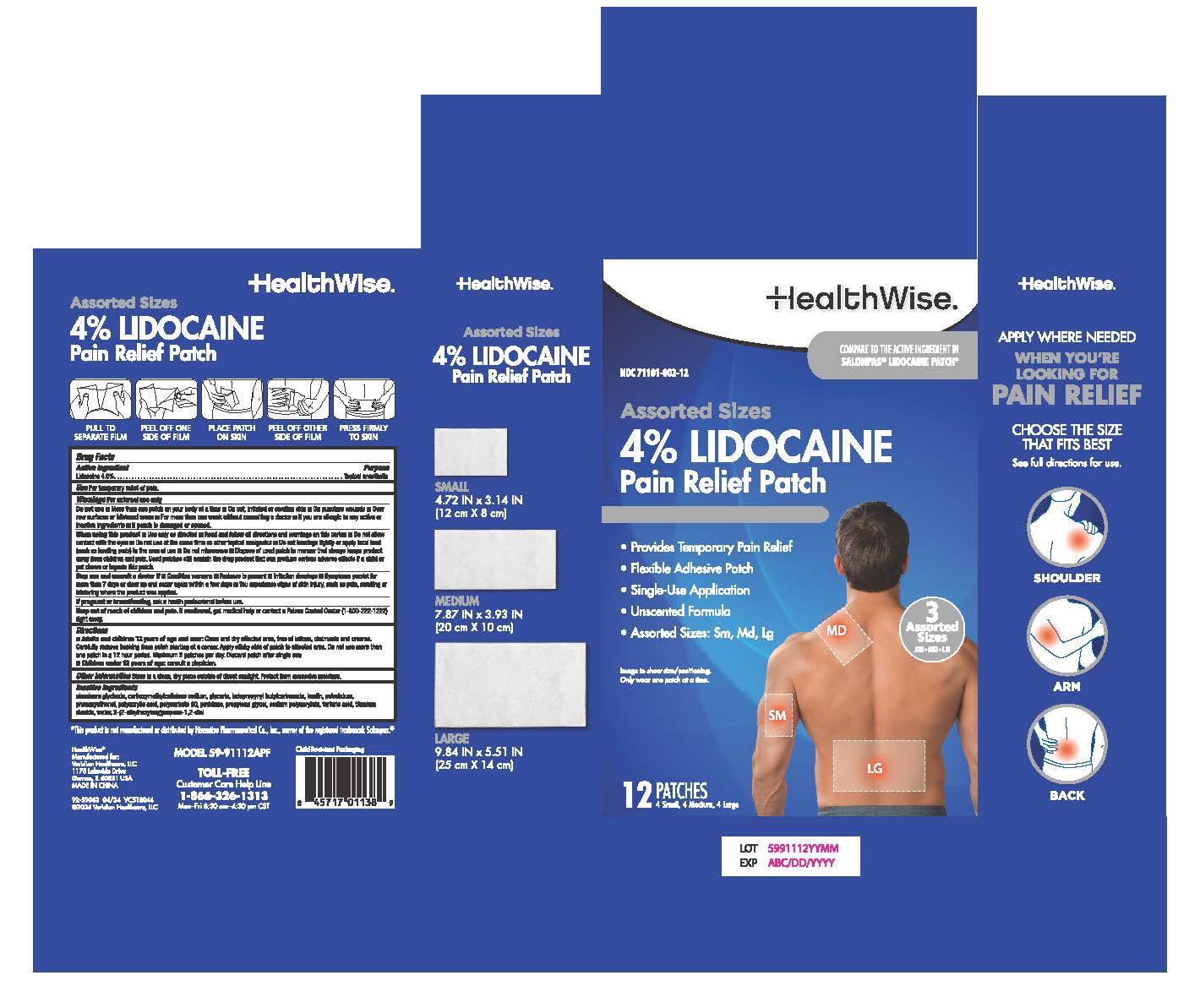
HEALTHWISE PAIN RELIEF LIDOCAINE PATCH- lidocaine 4% patch
HealthWise Pain Relief Lidocaine Patch by
Drug Labeling and Warnings
HealthWise Pain Relief Lidocaine Patch by is a Otc medication manufactured, distributed, or labeled by Veridian Healthcare, Foshan Aqua Gel Biotech Co., Ltd.,.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Use
-
Warnings
For External Use Only
Do not Use
- More than one patch on your body at a time
- On cut, irritated or swollen skin
- On puncture wounds
- For more than one week without consulting a doctor
- If you are allergic to any active or inactive ingredients
- If pouch is damaged or opened.
When using this product
- Use only as directed
- Read and follow all directions and warnings on this carton
- Do not allow contact with the eyes
- Do not use at the same time as other topical anesthetics
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use
- Do not microwave
- Dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects is a child or pet chews or ingests this patch.
Stop use and consult a doctor if
- Condition worsen
- Redness is present
- Irritation develops
- Symptoms persist for more than 7 days or clear up and occur again within a few days
- You experience signs of skin injury, such as pain, swelling, or blistering where the product was applied.
- Directions
- Other Information
- Inactive Ingredients
- HealthWise Pain Relief 4% Lidocaine Patch
-
INGREDIENTS AND APPEARANCE
HEALTHWISE PAIN RELIEF LIDOCAINE PATCH
lidocaine 4% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71101-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) PETROLATUM (UNII: 4T6H12BN9U) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) WATER (UNII: 059QF0KO0R) POLYACRYLIC ACID (8000 MW) (UNII: 73861X4K5F) PHENOXYETHANOL (UNII: HIE492ZZ3T) KAOLIN (UNII: 24H4NWX5CO) TARTARIC ACID (UNII: W4888I119H) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71101-002-25 25 in 1 CARTON 07/01/2023 1 1 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC: 71101-002-12 12 in 1 CARTON 04/01/2024 2 1 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/01/2023 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd.,. 529128763 manufacture(71101-002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.