ChapStick® Hydration Lock Day and Night
ChapStick Hydration Lock Day and Night by
Drug Labeling and Warnings
ChapStick Hydration Lock Day and Night by is a Otc medication manufactured, distributed, or labeled by Wyeth Consumer Healthcare LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CHAPSTICK HYDRATION LOCK DAY AND NIGHT- white petrolatum, octinoxate, oxybenzone
Wyeth Consumer Healthcare LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ChapStick® Hydration Lock Day and Night
Uses
- helps prevent and temporarily protects chafed, chapped or cracked lips
- helps prevent sunburn
- helps prevent and protect from the drying effects of wind and cold weather
Warnings
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
Other information
- store at 20-25°C (68-77°F)
- protect this product from excessive heat and direct sun.
Inactive ingredients
aloe barbadensis leaf extract, carnauba wax, cetyl alcohol, fragrance, isocetyl stearate, isopropyl lanolate, isopropyl myristate, lanolin, methylparaben, mineral oil, paraffin, propylparaben, tocopheryl linoleate/oleate, vitamin E acetate, white wax
Questions or comments?
call weekdays from 9 AM to 5 PM EST at 1-877-227-3421
For most recent product information, visit www.chapstick.com
ChapStick® Night
Ingredients
helianthus annuus (sunflower) seed oil, jojoba esters, euphorbia cerifera (candelilla) wax, cocos nucifera (coconut) oil, beeswax, copernicia cerifera (carnauba) wax, tocopheryl acetate, mangifera indica (mango) seed butter, butyrospermum parkii (shea butter), octyldodecanol, flavor, persea gratissima (avocado) oil, olea europaea (olive) fruit oil, calophyllum tacamahaca seed oil, tocopherol, hydrogenated soybean oil, rubus idaeus (raspberry) seed oil, glyceryl stearate
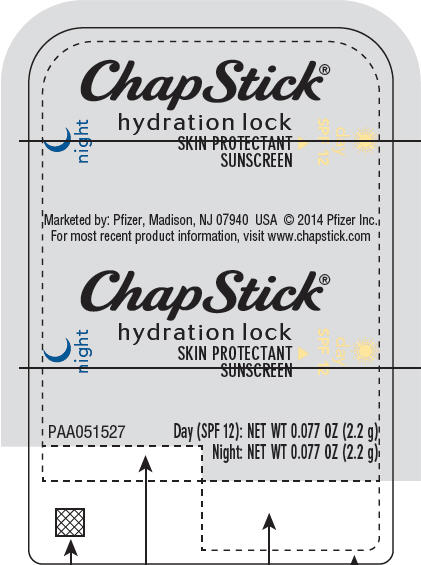
PRINCIPAL DISPLAY PANEL - 2.2 g Cylinder Label
ChapStick®
night
hydration lock
SKIN PROTECTANT
SUNSCREEN
day
SPF 12
PAA051527
Day (SPF 12): NET WT 0.077 OZ (2.2 g)
Night: NET WT 0.077 OZ (2.2 g)
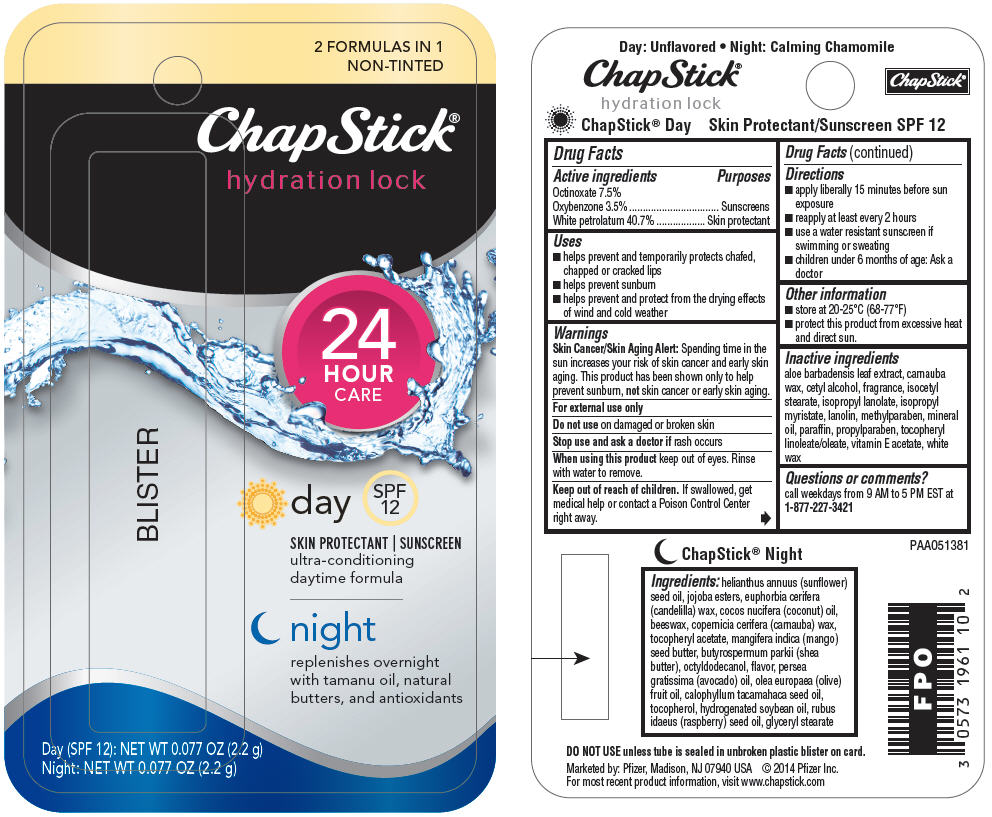
PRINCIPAL DISPLAY PANEL - 2.2 g Blister Pack Label
2 FORMULAS IN 1
NON-TINTED
ChapStick®
hydration lock
24
HOUR
CARE
BLISTER
day
SPF
12
SKIN PROTECTANT | SUNSCREEN
ultra-conditioning
daytime formula
night
replenishes overnight
with tamanu oil, natural
butters, and antioxidants
Day (SPF 12): NET WT 0.077 OZ (2.2 g)
Night: NET WT 0.077 OZ (2.2 g)
| CHAPSTICK HYDRATION LOCK DAY AND NIGHT
white petrolatum, octinoxate, oxybenzone kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Wyeth Consumer Healthcare LLC (828831730) |