STERILE ALCOHOL PREP PADS by PurCel Labs LLC / Zhejiang Qimei Cosmetics Co., Ltd.
STERILE ALCOHOL PREP PADS by
Drug Labeling and Warnings
STERILE ALCOHOL PREP PADS by is a Otc medication manufactured, distributed, or labeled by PurCel Labs LLC, Zhejiang Qimei Cosmetics Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
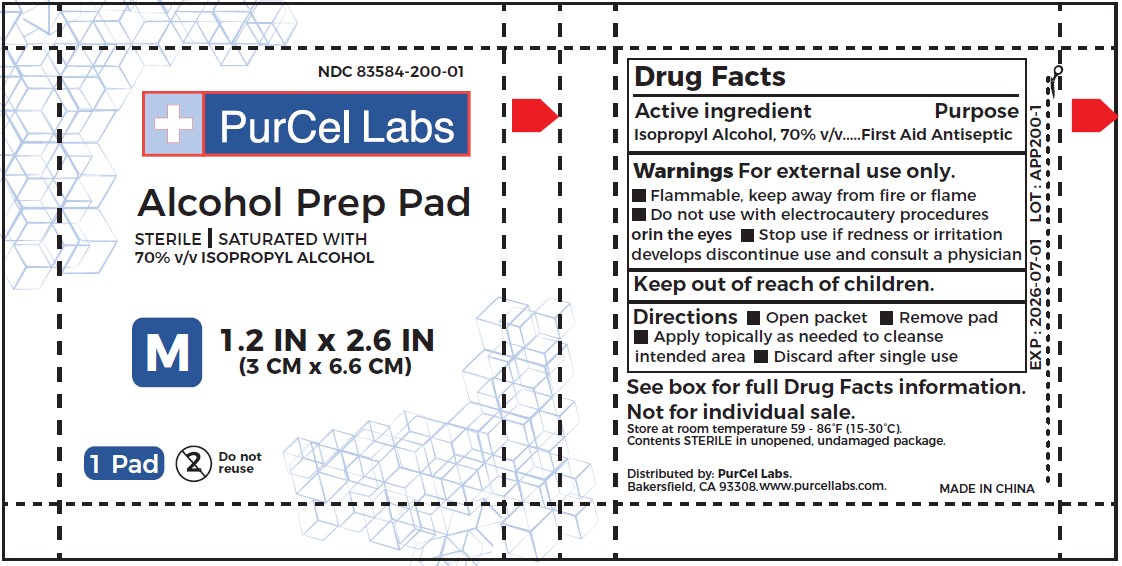
STERILE ALCOHOL PREP PADS- isopropyl alcohol cloth
PurCel Labs LLC
----------
Directions
Open packet
Apply topically as needed to cleanse
Remove pad
intended area Discard after single use
| STERILE ALCOHOL PREP PADS
isopropyl alcohol cloth |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PurCel Labs LLC (127672531) |
| Registrant - Zhejiang Qimei Cosmetics Co., Ltd. (709887693) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Qimei Cosmetics Co., Ltd. | 709887693 | manufacture(83584-200) | |
Revised: 10/2024
Document Id: 23daba8c-09e2-35a1-e063-6294a90ab50e
Set id: 01dcfd14-7a1f-98fa-e063-6294a90a0336
Version: 3
Effective Time: 20241006
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.