Identipak- BYHUMANKIND: Cinnamon
Kindfill by
Drug Labeling and Warnings
Kindfill by is a Otc medication manufactured, distributed, or labeled by Identipak, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KINDFILL- cinnamon toothpaste tablets tablet, chewable
Identipak, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Identipak- BYHUMANKIND: Cinnamon
Warnings
- When using this product, if irritation or sensitivity occurs discontinue use.
- Keep out of the reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Adults and children 6 years of age and older; chew one tablet until crushed, wet toothbrush, brush for 2 minutes. Spit out.
- Do not swallow the tablet.
- Children under 6 years of age should be supervised when using this product.
| KINDFILL
cinnamon toothpaste tablets tablet, chewable |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Identipak, Inc (942862350) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Identipak, Inc | 942862350 | repack(64796-322) | |
Revised: 8/2023
Document Id: 02953481-629c-6af9-e063-6394a90a1422
Set id: 029534c8-8dd4-7d53-e063-6394a90a63b4
Version: 1
Effective Time: 20230810
Trademark Results [Kindfill]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KINDFILL 88059393 not registered Live/Pending |
By Humankind, Inc. 2018-07-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
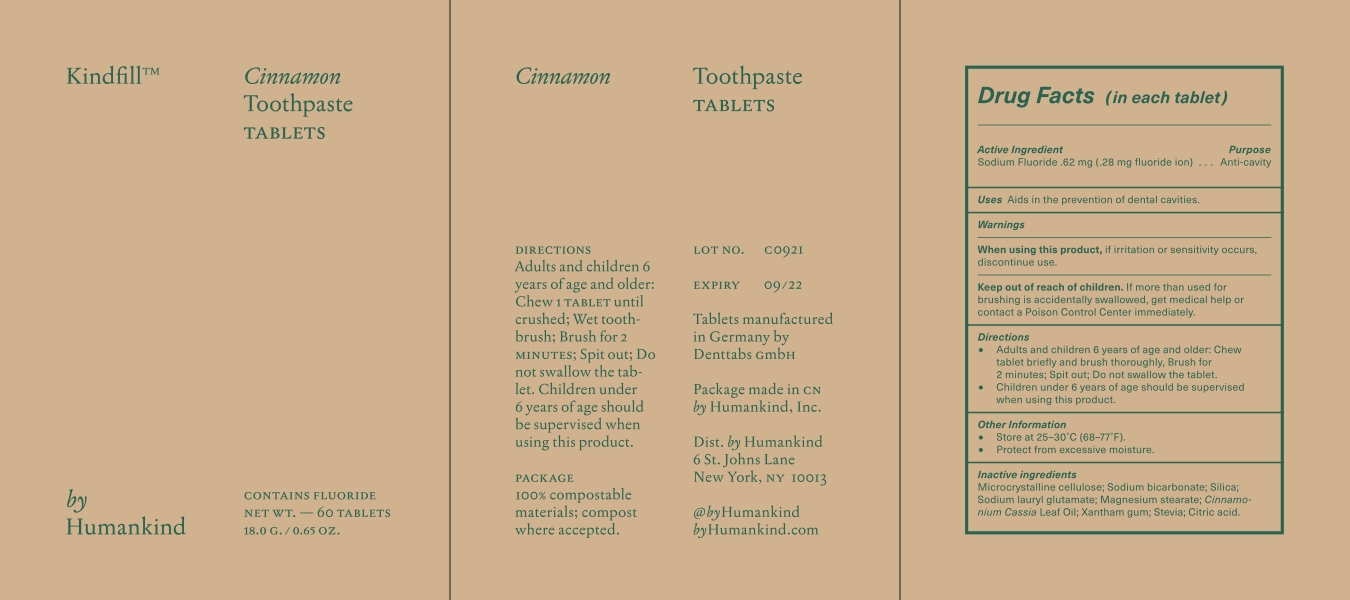 60 in Each Box NDC:
60 in Each Box NDC: