REZUROCK ®(belumosudil) tablets, for oral use (Packaged by Pharma Packaging Solutions, LLC dba Tjoapack LLC) These highlights do not include all the information needed to use REZUROCK safely and effectively. See full prescribing information for REZUROCK. Initial U.S. Approval: 2021
Rezurock by
Drug Labeling and Warnings
Rezurock by is a Prescription medication manufactured, distributed, or labeled by Pharma Packaging Solutions, LLC dba Tjoapack LLC, Gregory Pharmaceutical Holdings, Inc., dba UPM Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REZUROCK- belumosudil tablet
Pharma Packaging Solutions, LLC dba Tjoapack LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
REZUROCK ®(belumosudil) tablets, for oral use
(Packaged by Pharma Packaging Solutions, LLC dba Tjoapack LLC) These highlights do not include all the information needed to use REZUROCK safely and effectively. See full prescribing information for REZUROCK. Initial U.S. Approval: 2021 INDICATIONS AND USAGEREZUROCK is a kinase inhibitor indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy. ( 1) DOSAGE FORMS AND STRENGTHSTablet: 200 mg. ( 3) CONTRAINDICATIONSNone. ( 4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSThe most common (≥20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension. ( 6.1) To report SUSPECTED ADVERSE REACTIONS, contact Kadmon Pharmaceuticals, LLC at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSStrong CYP3A Inducers: Increase REZUROCK dosage to 200 mg twice daily. ( 7.1) Proton Pump Inhibitors: Increase REZUROCK dosage to 200 mg twice daily. ( 7.1) See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 4/2023 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
REZUROCK is indicated for the treatment of adult and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior lines of systemic therapy.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of REZUROCK is 200 mg given orally once daily until progression of chronic GVHD that requires new systemic therapy.
Instruct the patient on the following:
- Swallow REZUROCK tablets whole. Do not cut, crush, or chew tablets.
- Take REZUROCK with a meal at approximately the same time each day [see Clinical Pharmacology (12.3)] .
- If a dose of REZUROCK is missed, instruct the patient to not take extra doses to make up the missed dose.
Treatment with REZUROCK has not been studied in patients with pre-existing severe renal or hepatic impairment. For patients with pre-existing severe renal or hepatic impairment, consider the risks and potential benefits before initiating treatment with REZUROCK [see Clinical Pharmacology (12.3)] .
2.2 Dose Modifications for Adverse Reactions
Monitor total bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) at least monthly.
Modify the REZUROCK dosage for adverse reactions as per Table 1.
| Adverse Reaction | Severity * | REZUROCK Dosage Modifications |
|---|---|---|
|
|
||
| Hepatotoxicity [see Adverse Reactions (6.1)] | Grade 3 AST or ALT (5× to 20× ULN) or Grade 2 bilirubin (1.5× to 3× ULN) | Hold REZUROCK until recovery of bilirubin, AST and ALT to Grade 0–1, then resume REZUROCK at the recommended dose. |
| Grade 4 AST or ALT (more than 20× ULN) or Grade ≥3 bilirubin (more than 3× ULN) | Discontinue REZUROCK permanently. | |
| Other adverse reactions [see Adverse Reactions (6.1)] | Grade 3 | Hold REZUROCK until recovery to Grade 0–1, then resume REZUROCK at the recommended dose level. |
| Grade 4 | Discontinue REZUROCK permanently. | |
2.3 Dosage Modification Due to Drug Interactions
Strong CYP3A Inducers
Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with strong CYP3A inducers [see Drug Interactions (7.1)] .
Proton Pump Inhibitors
Increase the dosage of REZUROCK to 200 mg twice daily when coadministered with proton pump inhibitors [see Drug Interactions (7.1)] .
3 DOSAGE FORMS AND STRENGTHS
Each 200 mg tablet is a pale yellow film-coated oblong tablet debossed with "KDM" on one side and "200" on the other side.
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, REZUROCK can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of belumosudil to pregnant rats and rabbits during the period organogenesis caused adverse developmental outcomes including embryo-fetal mortality and malformations at maternal exposures (AUC) less than those in patients at the recommended dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with REZUROCK and for at least one week after the last dose [see Use in Specific Populations (8.1, 8.3), Nonclinical Toxicology (13.1)] .
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely variable conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared with rates of clinical trials of another drug and may not reflect the rates observed in practice.
Chronic Graft versus Host Disease
In two clinical trials (Study KD025-213 and Study KD025-208), 83 adult patients with chronic GVHD were treated with REZUROCK 200 mg once daily [see Clinical Studies (14.1)] . The median duration of treatment was 9.2 months (range 0.5 to 44.7 months).
Fatal adverse reaction was reported in one patient with severe nausea, vomiting, diarrhea and multi-organ failure.
Permanent discontinuation of REZUROCK due to adverse reactions occurred in 18% of patients. The adverse reactions which resulted in permanent discontinuation of REZUROCK in >3% of patients included nausea (4%). Adverse reactions leading to dose interruption occurred in 29% of patients. The adverse reactions leading to dose interruption in ≥2% were infections (11%), diarrhea (4%), and asthenia, dyspnea, hemorrhage, hypotension, liver function test abnormal, nausea, pyrexia, edema, and renal failure with (2% each).
The most common (≥20%) adverse reactions, including laboratory abnormalities, were infections, asthenia, nausea, diarrhea, dyspnea, cough, edema, hemorrhage, abdominal pain, musculoskeletal pain, headache, phosphate decreased, gamma glutamyl transferase increased, lymphocytes decreased, and hypertension.
Table 2summarizes the nonlaboratory adverse reactions.
| Adverse Reaction | REZUROCK
200 mg once daily (N=83) |
|
|---|---|---|
| All Grades (%) | Grades 3–4 (%) | |
|
|
||
| Infections and infestations | ||
| Infection (pathogen not specified) * | 53 | 16 |
| Viral infection † | 19 | 4 |
| Bacterial infection ‡ | 16 | 4 |
| General disorders and administration site conditions | ||
| Asthenia § | 46 | 4 |
| Edema ¶ | 27 | 1 |
| Pyrexia | 18 | 1 |
| Gastrointestinal | ||
| Nausea # | 42 | 4 |
| Diarrhea | 35 | 5 |
| Abdominal pain Þ | 22 | 1 |
| Dysphagia | 16 | 0 |
| Respiratory, thoracic and mediastinal | ||
| Dyspnea ß | 33 | 5 |
| Cough à | 30 | 0 |
| Nasal congestion | 12 | 0 |
| Vascular | ||
| Hemorrhage è | 23 | 5 |
| Hypertension | 21 | 7 |
| Musculoskeletal and connective tissue | ||
| Musculoskeletal pain ð | 22 | 4 |
| Muscle spasm | 17 | 0 |
| Arthralgia | 15 | 2 |
| Nervous system | ||
| Headache ø | 21 | 0 |
| Metabolism and nutrition | ||
| Decreased appetite | 17 | 1 |
| Skin and subcutaneous | ||
| Rash ý | 12 | 0 |
| Pruritus £ | 11 | 0 |
Table 3summarizes the laboratory abnormalities in REZUROCK.
| REZUROCK
200 mg once daily |
|||
|---|---|---|---|
| Grade 0–1
Baseline | Grade 2–4
Max Post | Grade 3–4
Max Post |
|
| Parameter | (N) | (%) | (%) |
| Chemistry | |||
| Phosphate decreased | 76 | 28 | 7 |
| Gamma Glutamyl Transferase increased | 47 | 21 | 11 |
| Calcium decreased | 82 | 12 | 1 |
| Alkaline Phosphatase increased | 80 | 9 | 0 |
| Potassium increased | 82 | 7 | 1 |
| Alanine Aminotransferase increased | 83 | 7 | 2 |
| Creatinine increased | 83 | 4 | 0 |
| Hematology | |||
| Lymphocytes decreased | 62 | 29 | 13 |
| Hemoglobin decreased | 79 | 11 | 1 |
| Platelets decreased | 82 | 10 | 5 |
| Neutrophil Count decreased | 83 | 8 | 4 |
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on REZUROCK
Strong CYP3A Inducers
Coadministration of REZUROCK with strong CYP3A inducers decreases belumosudil exposure [see Clinical Pharmacology (12.3)] , which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK when coadministered with strong CYP3A inducers [see Dosage and Administration (2.3)] .
Proton Pump Inhibitors
Coadministration of REZUROCK with proton pump inhibitors decreases belumosudil exposure [see Clinical Pharmacology (12.3)] , which may reduce the efficacy of REZUROCK. Increase the dosage of REZUROCK when coadministered with proton pump inhibitors [see Dosage and Administration (2.3)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and the mechanism of action [see Clinical Pharmacology (12.1)] , REZUROCK can cause fetal harm when administered to pregnant women. There are no available human data on REZUROCK use in pregnant women to evaluate for a drug-associated risk. In animal reproduction studies, administration of belumosudil to pregnant rats and rabbits during the period of organogenesis resulted in adverse developmental outcomes, including alterations to growth, embryo-fetal mortality, and embryo-fetal malformations at maternal exposures (AUC) approximately ≥3- (rat) and ≥0.07 (rabbit) times the human exposure (AUC) at the recommended dose (see Animal data). Advise pregnant women and females of reproductive potential of the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal data
Embryo-fetal development studies were conducted in rats with administration of belumosudil to pregnant animals during the period of organogenesis at oral doses of 25, 50, 150, and 300 mg/kg/day in a pilot study and doses of 15, 50, and 150 mg/kg/day in a pivotal study. In the pilot study, maternal toxicity and embryo-fetal developmental effects were observed. Maternal toxicity (reduced body weight gain) occurred at 150 and 300 mg/kg/day doses. Increased post-implantation loss occurred at 50 and 300 mg/kg/day. Fetal-malformations were observed at ≥50 mg/kg/day and included absence of anus and tail, omphalocele, and dome shaped head. The exposure (AUC) at 50 mg/kg/day in rats is approximately 3 times the human exposure at the recommended dose of 200 mg.
In an embryo-fetal developmental study in rabbits, pregnant animals administered oral doses of belumosudil at 50, 125, and 225 mg/kg/day during the period of organogenesis resulted in maternal toxicity and embryo-fetal developmental effects. Maternal toxicity (body weight loss and mortality) was observed at doses ≥125 mg/kg/day. Embryo-fetal effects were observed at doses ≥50 mg/kg/day and included spontaneous abortion, increased post-implantation loss, decreased percentage of live fetuses, malformations, and decreased fetal body weight. Malformations included those in the tail (short), ribs (branched, fused or deformed), sternebrae (fused), and neural arches (fused, misaligned, and deformed). The exposure (AUC) at 50 mg/kg/day in rabbits is approximately 0.07 times the human exposure at the recommended dose of 200 mg.
8.2 Lactation
Risk Summary
There are no data available on the presence of belumosudil or its metabolites in human milk or the effects on the breastfed child, or milk production. Because of the potential for serious adverse reactions from belumosudil in the breastfed child, advise lactating women not to breastfeed during treatment with REZUROCK and for at least one week after the last dose.
8.3 Females and Males of Reproductive Potential
REZUROCK can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] .
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating treatment with REZUROCK.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with REZUROCK and for at least one week after the last dose of REZUROCK. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be informed of the potential hazard to a fetus.
Infertility
Females
Based on findings from rats, REZUROCK may impair female fertility. The effect on fertility is reversible [see Nonclinical Toxicology (13.1)].
Males
Based on findings from rats and dogs, REZUROCK may impair male fertility. The effects on fertility are reversible [see Nonclinical Toxicology (13.1)] .
8.4 Pediatric Use
The safety and effectiveness of REZUROCK have been established in pediatric patients 12 years and older. Use of REZUROCK in this age group is supported by evidence from adequate and well-controlled studies of REZUROCK in adults with additional population pharmacokinetic data demonstrating that age and body weight had no clinically meaningful effect on the pharmacokinetics of drug substance, that the exposure of drug substance is expected to be similar between adults and pediatric patients age 12 years and older, and that the course of disease is sufficiently similar in adult and pediatric patients to allow extrapolation of data in adults to pediatric patients.
The safety and effectiveness of REZUROCK in pediatric patients less than 12 years old have not been established.
11 DESCRIPTION
Belumosudil is a kinase inhibitor. The active pharmaceutical ingredient is belumosudil mesylate with the molecular formula C 27H 28N 6O 5S and the molecular weight is 548.62 g/mol. The chemical name for belumosudil mesylate is 2-{3-[4-(1 H-indazol-5-ylamino)-2-quinazolinyl]phenoxy}- N-(propan-2-yl) acetamide methanesulfonate (1:1). The chemical structure is as follows:
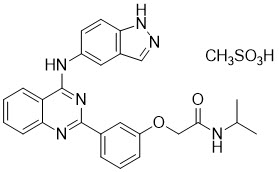
Belumosudil mesylate is a yellow powder that is practically insoluble in water, slightly soluble in methanol and DMF and soluble in DMSO.
REZUROCK tablets are for oral administration. Each tablet contains 200 mg of the free base equivalent to 242.5 mg of belumosudil mesylate. The tablet also contains the following inactive ingredients: microcrystalline cellulose, hypromellose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate.
The tablet film consists of polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide and yellow iron oxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Belumosudil is an inhibitor of rho-associated, coiled-coil containing protein kinase (ROCK) which inhibits ROCK2 and ROCK1 with IC 50values of approximately 100 nM and 3 µM, respectively. Belumosudil down-regulated proinflammatory responses via regulation of STAT3/STAT5 phosphorylation and shifting Th17/Treg balance in ex-vivo or in vitro-human T cell assays. Belumosudil also inhibited aberrant pro-fibrotic signaling, in vitro. In vivo, belumosudil demonstrated activity in animal models of chronic GVHD.
12.2 Pharmacodynamics
Belumosudil exposure-response relationships and the time course of pharmacodynamic response are not established.
12.3 Pharmacokinetics
The following pharmacokinetic parameters are presented for chronic GVHD patients administered belumosudil 200 mg once daily, unless otherwise specified. The mean (% coefficient of variation, %CV) steady-state AUC and C maxof belumosudil was 22,700 (48%) h∙ng/mL and 2390 (44%) ng/mL, respectively. Belumosudil C maxand AUC increased in an approximately proportional manner over a dosage range of 200 and 400 mg (1 to 2 times once daily recommended dosage). The accumulation ratio of belumosudil was 1.4.
Absorption
Median T maxof belumosudil at steady state was 1.26 to 2.53 hours following administration of 200 mg once daily or twice daily in patients. The mean (%CV) bioavailability was 64% (17%) following a single belumosudil dose in healthy subjects.
Effect of food
Belumosudil C maxand AUC increased 2.2 times and 2 times, respectively, following administration of a single belumosudil dose with a high-fat and high-calorie meal (800 to 1,000 calories with approximately 50% of total caloric content of the meal from fat) compared to the fasted state in healthy subjects. Median T maxwas delayed 0.5 hours.
Distribution
The geometric mean volume of distribution after a single dose of belumosudil in healthy subjects was 184 L (geo CV% 67.7%).
Belumosudil binding to human serum albumin and human α 1-acid glycoprotein was 99.9% and 98.6%, respectively, in vitro.
Elimination
The mean (%CV) elimination half-life of belumosudil was 19 hours (39%), and clearance was 9.83 L/hours (46%) in patients.
Specific Populations
No clinically significant differences in belumosudil pharmacokinetics were observed with regard to age (18 to 77 years), sex, weight (38.6 to 143 kg), or mild to moderate renal impairment (eGFR ≥60 and <90 mL/min/1.72m 2to eGFR ≥30 and <60 mL/min/1.72m 2). The effect of severe renal impairment on the pharmacokinetics of belumosudil has not been studied.
Drug Interaction Studies
Clinical studies and model-informed approaches
Effects of other drugs on Belumosudil
Strong Cytochrome P450 (CYP) 3A Inhibitors: There was no clinically meaningful effect on belumosudil exposure when coadministered with itraconazole in healthy subjects.
Strong CYP3A Inducers: Coadministration of rifampin decreased belumosudil C maxby 59% and AUC by 72% in healthy subjects.
Effects of Belumosudil on other drugs
CYP3A Substrates: Coadministration of belumosudil is predicted to increase midazolam (a sensitive CYP3A substrate) C maxand AUC approximately 1.3- and 1.5-fold, respectively.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Belumosudil was not genotoxic in an in vitrobacterial mutagenicity (Ames) assay, in vitrochromosome aberration assay in human peripheral blood lymphocytes (HPBL) or an in vivorat bone marrow micronucleus assay.
Impairment of Fertility
In a combined male and female rat fertility study, belumosudil-treated male animals were mated with untreated females, or untreated males were mated with belumosudil-treated females. Belumosudil was administered orally at doses of 50, 150 or 275 mg/kg/day to male rats 70 days prior to and throughout the mating period, and to female rats 14 days prior to mating and up to Gestation Day 7. At the dose of 275 mg/kg/day, adverse findings in female rats (treated with belumosudil or untreated but mated with treated males) included increased pre- or post-implantation loss and decreased number of viable embryos. Administration of belumosudil to male rats at a dose of 275 mg/kg/day resulted in abnormal sperm findings (reduced motility, reduced count, and increased percentage of abnormal sperm), and testes/epididymis organ changes (reduced weight and degeneration). Fertility was reduced in both treated males or females at the 275 mg/kg/day dose and reached statistical significance in males. Adverse changes in male and female reproductive organs also occurred in general toxicology studies; findings included spermatozoa degeneration at a belumosudil dose of 35 mg/kg/day in dogs and decreased follicular development in ovaries at 275 mg/kg/day in rats. Changes were partially or fully reversed during the recovery period. The exposure (AUC) at the doses of 35 mg/kg/day in dogs, and 275 mg/kg/day in rats is 0.5 times and 8–9 times, respectively, the clinical exposure at the recommended dose of 200 mg daily.
14 CLINICAL STUDIES
14.1 Chronic Graft versus Host Disease
Study KD025-213 (NCT03640481) was a randomized, open-label, multicenter study of REZUROCK for treatment of patients with chronic GVHD who had received 2 to 5 prior lines of systemic therapy and required additional treatment. Patients were excluded from the studies if platelets were <50 × 10 9/L; absolute neutrophil count <1.5 × 10 9/L; AST or ALT >3 × ULN; total bilirubin >1.5 × ULN; QTc(F) >480 ms; eGFR <30 mL/min/1.73 m 2; or FEV1 ≤39%. There were 66 patients treated with REZUROCK 200 mg taken orally once daily. Concomitant treatment with supportive care therapies for chronic GVHD was permitted. Concomitant treatment with GVHD prophylaxis and standard care systemic chronic GVHD therapies was permitted as long as the subject has been on a stable dose for at least 2 weeks prior to study. Initiation of new systemic chronic GVHD therapy while on study was not permitted.
Demographics and baseline characteristics are summarized in Table 4.
| REZUROCK
200 mg once daily (N=65) |
|
|---|---|
|
|
|
| Age, Median, Years (minimum, maximum) | 53 (21, 77) |
| Age ≥65 Years, n (%) | 17 (26) |
| Male, n (%) | 42 (65) |
| Race, n (%) | |
| White | 54 (83) |
| Black | 6 (9) |
| Other or Not Reported | 5 (8) |
| Median (range) time (months) from Chronic GVHD Diagnosis | 25.3 (1.9, 162.4) |
| ≥4 Organs Involved, n (%) | 31 (48) |
| Median (range) Number of Prior Lines of Therapy | 3 (2, 6) |
| Number of Prior Lines of Therapy, n (%) | |
| 2 | 23 (35) |
| 3 | 12 (19) |
| 4 | 15 (23) |
| ≥5 | 15 (23) |
| Prior chronic GVHD treatment with ibrutinib, n (%) | 21 (32) |
| Prior chronic GVHD treatment with ruxolitinib, n (%) | 20 (31) |
| Refractory to Last Therapy, n (% *) | 43/55 (78) |
| Severe chronic GVHD, n (%) | 46 (71) |
| Median (range) Global Severity Rating | 7 (2, 9) |
| Median (range) Lee Symptom Scale Score at baseline | 27 (7, 56) |
| Median (range) Corticosteroid dose at baseline (PE/kg) † | 0.19 (0.03, 0.95) |
The efficacy of REZUROCK was based on overall response rate (ORR) through Cycle 7 Day 1 where overall response included complete response or partial response according to the 2014 NIH Response Criteria. The ORR results are presented in Table 5. The ORR was 75% (95% CI: 63, 85). The median duration of response, calculated from first response to progression, death, or new systemic therapies for chronic GVHD, was 1.9 months (95% CI: 1.2, 2.9). The median time to first response was 1.8 months (95% CI: 1.0, 1.9). In patients who achieved response, no death or new systemic therapy initiation occurred in 62% (95% CI: 46, 74) of patients for at least 12 months since response.
| REZUROCK
200 mg once daily (N=65) |
|
|---|---|
|
|
|
| Overall Response Rate (ORR) | 49 (75%) |
| 95% Confidence Interval * | (63%, 85%) |
| Complete Response | 4 (6%) |
| Partial Response | 45 (69%) |
ORR results were supported by exploratory analyses of patient-reported symptom bother which showed at least a 7-point decrease in the Lee Symptom Scale summary score through Cycle 7 Day 1 in 52% (95% CI: 40, 65) of patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
REZUROCK 200 mg tablets are supplied as pale yellow film-coated oblong tablets containing 200 mg of belumosudil (equivalent to 242.5 mg belumosudil mesylate). Each tablet is debossed with "KDM" on one side and "200" on the other side and is packaged as follows:
- 200 mg tablets in 30 count bottle: NDC: 75929-174-03
Store at room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Dispense to patient in original container only. Store in original container to protect from moisture. Replace cap securely each time after opening. Do not discard desiccant.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryo-fetal Toxicity:
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
- Advise females of reproductive potential to use effective contraceptive during treatment with REZUROCK and for at least one week after the last dose [see Warnings and Precautions (5.1)].
- Advise males with female partners of reproductive potential to use effective contraceptive during treatment with REZUROCK and for at least one week after the last dose [see Use in Specific Populations (8.3)] .
Lactation
- Advise women not to breastfeed during treatment with REZUROCK and for at least one week after the last dose [see Use in Specific Populations (8.2)] .
Infertility
- Advise males and females of reproductive potential that REZUROCK may impair fertility [see Use in Specific Populations (8.3)].
Administration
- Inform patients to take REZUROCK orally once daily with food according to their physician's instructions and that the oral dosage (tablets) should be swallowed whole with a glass of water, without cutting, crushing or chewing the tablets approximately the same time each day [see Dosage and Administration (2.1)].
- Advise patients that in the event of a missed daily dose of REZUROCK, it should be taken as soon as possible on the same day with a return to the normal schedule the following day. Patients should not take extra doses to make up the missed dose [see Dosage and Administration (2.1)].
Drug Interactions
- Advise patients to inform their health care providers of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products [see Drug Interactions (7)] .
Distributed and marketed by:
Kadmon Pharmaceuticals, LLC
Bridgewater, NJ 08807
A SANOFI COMPANY
1-800-633-1610
REZUROCK
®is a registered trademark of Kadmon Pharmaceuticals, LLC.
© 2023 Kadmon Pharmaceuticals, LLC. All rights reserved.
For patent information: https://www.sanofi.us/en/products-and-resources/patents
| This Patient Information has been approved by the U.S. Food and Drug Administration | Issued: July 2022 | |
| PATIENT INFORMATION
REZUROCK (REZ-ur-ok) (belumosudil) tablets |
||
| What is REZUROCK? | ||
| REZUROCK is a prescription medicine used to treat adults and children 12 years of age and older with chronic graft-versus-host disease (chronic GVHD) after you have received at least 2 prior treatments (systemic therapy) and they did not work.
It is not known if REZUROCK is safe and effective in children less than 12 years old. |
||
Before taking REZUROCK, tell your healthcare provider about all of your medical conditions, including if you:
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
||
How should I take REZUROCK?
|
||
| What are the possible side effects of REZUROCK?
The most common side effects of REZUROCK include: |
||
|
|
|
| Your healthcare provider may change your dose of REZUROCK, temporarily stop, or permanently stop treatment with REZUROCK if you have certain side effects.
REZUROCK may affect fertility in males and females. Talk to your healthcare provider if this is a concern for you. These are not all the possible side effects of REZUROCK. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Kadmon Pharmaceuticals, LLC at 1-800-633-1610. |
||
How should I store REZUROCK?
|
||
| General information about the safe and effective use of REZUROCK.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use REZUROCK for a condition for which it was not prescribed. Do not give REZUROCK to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about REZUROCK that is written for health professionals. |
||
| What are the ingredients in REZUROCK?
Active ingredient: belumosudil mesylate Inactive ingredients: Tablet core: microcrystalline cellulose, hypromellose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate. Tablet coating: polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide and yellow iron oxide. Distributed and marketed by Kadmon Pharmaceuticals, LLC,Bridgewater, NJ 08807, A SANOFI COMPANY REZUROCK ®is a registered trademark of Kadmon Pharmaceuticals, LLC © 2022 Kadmon Pharmaceuticals, LLC. All rights reserved. For more information, call 1-800-633-1610 or go to www.REZUROCK.com. |
||
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle Carton
NDC: 75929-174-03
Rx only
REZUROCK
®
(belumosudil) tablets
200 mg
Swallow tablets whole. Do not cut,
crush, or chew the tablets.
30 Tablets
sanofi
| REZUROCK
belumosudil tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Pharma Packaging Solutions, LLC dba Tjoapack LLC (928861723) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gregory Pharmaceutical Holdings, Inc., dba UPM Pharmaceuticals | 081301372 | manufacture(75929-174) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharma Packaging Solutions, LLC dba Tjoapack LLC | 928861723 | pack(75929-174) | |
Trademark Results [Rezurock]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REZUROCK 90071349 not registered Live/Pending |
Kadmon Pharmaceuticals, LLC 2020-07-24 |
 REZUROCK 88401751 not registered Live/Pending |
Kadmon Pharmaceuticals, LLC 2019-04-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.