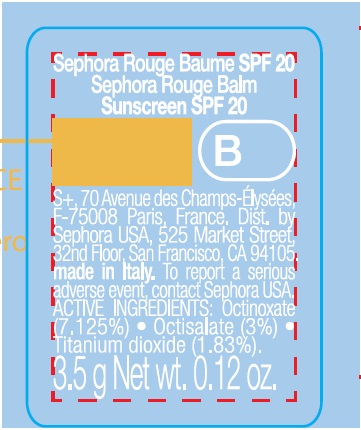
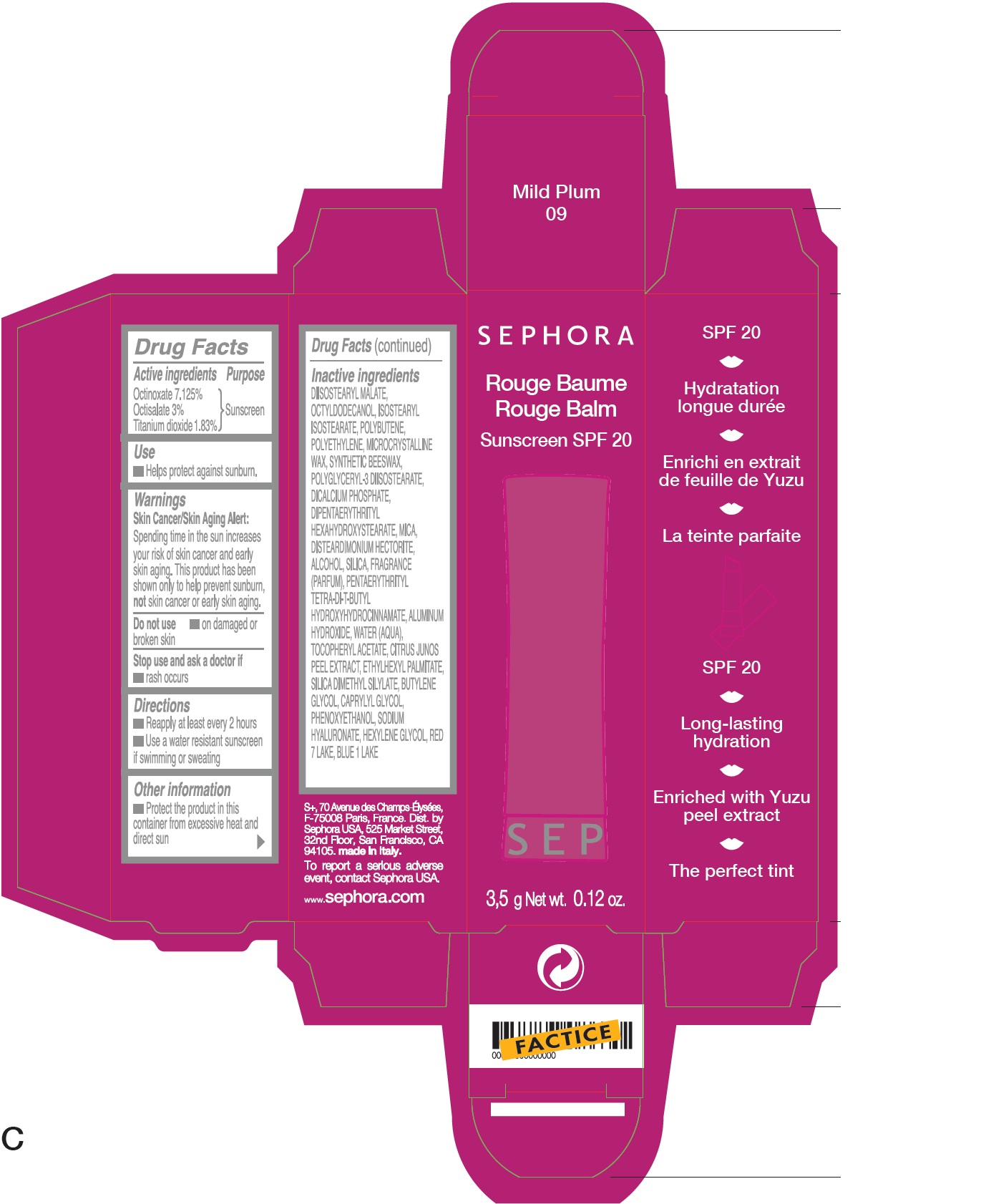
SEPHORA ROUGE BALM SUNSCREEN SPF20 B09-Mild Plum
SEPHORA ROUGE BALM SUNSCREEN SPF20 B09-Mild Plum by
Drug Labeling and Warnings
SEPHORA ROUGE BALM SUNSCREEN SPF20 B09-Mild Plum by is a Otc medication manufactured, distributed, or labeled by INTERCOS EUROPE SPA, Intercos Europe S.p.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SEPHORA ROUGE BALM SUNSCREEN SPF20 B09-MILD PLUM- octinoxate, octisalate, titanium dioxide lipstick
INTERCOS EUROPE SPA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SEPHORA ROUGE BALM SUNSCREEN SPF20 B09-Mild Plum
| SEPHORA ROUGE BALM SUNSCREEN SPF20 B09-MILD PLUM
octinoxate, octisalate, titanium dioxide lipstick |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - INTERCOS EUROPE SPA (338578473) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| INTERCOS EUROPE SPA | 339447428 | manufacture(63272-508) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intercos Europe S.p.A. | 438961310 | pack(63272-508) | |
Revised: 12/2019
Document Id: 9983d603-b7ca-5979-e053-2995a90adf8b
Set id: 029c3295-90ff-4af0-a456-33f736a1e630
Version: 4
Effective Time: 20191212
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.