PAIN RELIVEING PATCH- lidocaine 4% patch
Pain Reliveing Patch by
Drug Labeling and Warnings
Pain Reliveing Patch by is a Otc medication manufactured, distributed, or labeled by simple diagnostics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredient
- Purpose
- USE
- Warnings
-
When using this product
Use only as directed. Read and follow all directions and warnings on this carton. do not allow contact with the eyes
do not bandage tightly or apply local heat (such as heating pads) to the area of use do not use at the same time as other topical analcesics
dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch. - DO NOT USE:
- STOP USE AND ASK A DOCTOR IF:
- IF PREGNANT OR BREAST-FEEDING:
- Keep out of reach of children
-
Directions
Adults and children 12 years of age and older:
Wash and dry the affected area.
Peel off the backing film from one side of the patch carefully
Apply the exposed portion of the patch to the affected area.
Gently press the patch with your hand to fit the affected area completely.
Do not exercise vigorously during use to prevent the patch from falling off.
Use only 1 patch for a maximum of 8 hours.
Children under 12 years of age: Ask a doctor before use. - QUESTIONS OR COMMENTS:
- Other information
- Inactive ingredient
- PACKAGE LABEL. PRINCIPAL DISPLAY PANEL:
-
INGREDIENTS AND APPEARANCE
PAIN RELIVEING PATCH
lidocaine 4% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62379-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.4 g Inactive Ingredients Ingredient Name Strength KAOLIN (UNII: 24H4NWX5CO) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) POVIDONE K90 (UNII: RDH86HJV5Z) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62379-201-03 30 in 1 CASE 06/01/2023 1 NDC: 62379-201-02 5 in 1 BOX 1 NDC: 62379-201-01 1 in 1 BAG; Type 0: Not a Combination Product 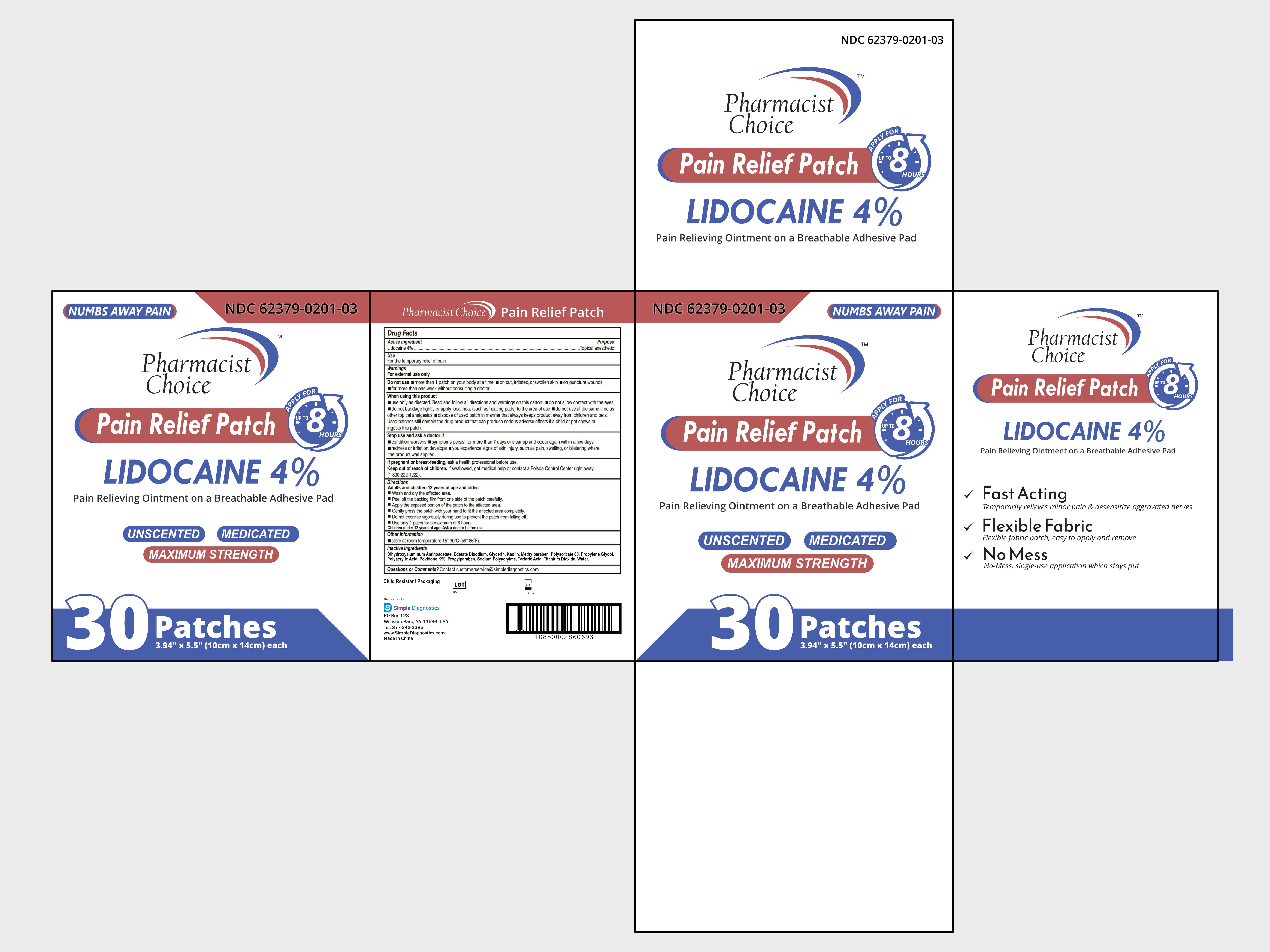
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2023 Labeler - simple diagnostics (004135503)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


