Amazon Lidocaine Patch Drug Facts
basic care lidocaine by
Drug Labeling and Warnings
basic care lidocaine by is a Otc medication manufactured, distributed, or labeled by Amazon.com Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BASIC CARE LIDOCAINE- lidocaine
Amazon.com Services LLC
----------
Amazon Lidocaine Patch Drug Facts
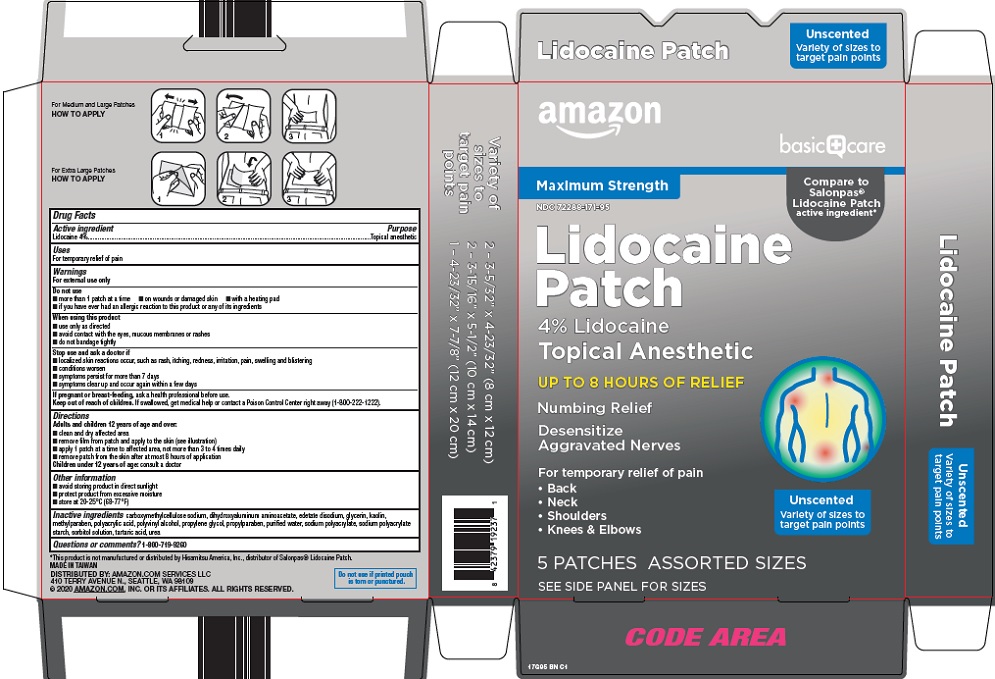
Warnings
For external use only
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you have ever had an allergic reaction to this product or any of its ingredients
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Directions
Adults and children 12 years of age and over:
- clean and dry affected area
- remove film from patch and apply to the skin (see illustration)
- apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- remove patch from the skin after at most 8 hours of application
Children under 12 years of age: consult a doctor
Other information
- avoid storing product in direct sunlight
- protect product from excessive moisture
- store at 20-25°C (68-77°F)
Inactive ingredients
carboxymethylcellulose sodium, dihydroxyaluminum aminoacetate, edetate disodium, glycerin, kaolin, methylparaben, polyacrylic acid, polyvinyl alcohol, propylene glycol, propylparaben, purified water, sodium polyacrylate, sodium polyacrylate starch, sorbitol solution, tartaric acid, urea
Package/Label Principal Display Panel
Maximum Strength
Compare to Salonpas® Lidocaine Patch active ingredient
Lidocaine Patch
4% Lidocaine
Topical Anesthetic
UP TO 8 HOURS OF RELIEF
Numbing Relief
Desensitize Aggravated Nerves
For temporary relief of pain
- Back
- Neck
- Shoulders
- Knees & Elbows
Unscented
Variety of sizes to target pain points
5 PATCHES ASSORTED SIZES
SEE SIDE PANEL FOR SIZES
| BASIC CARE LIDOCAINE
lidocaine kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Amazon.com Services LLC (128990418) |