Ultravate® (halobetasol propionate) Cream, 0.05% (halobetasol propionate) Ointment, 0.05% For Dermatological Use Only. Not for Ophthalmic Use. Rx only
Ultravate by
Drug Labeling and Warnings
Ultravate by is a Prescription medication manufactured, distributed, or labeled by Ranbaxy Laboratories Inc., Contract Pharmaceuticals Limited, Novartis Pharma Stein AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ULTRAVATE- halobetasol propionate cream
ULTRAVATE- halobetasol propionate ointment
Sun Pharmaceutical Industries, Inc.
Reference Label Set Id: 0a62f646-5c92-4cec-b50a-6b61ea83abcb
Reference Label Set Id: f1b22c22-6ee4-4e3e-8de4-8627a8c4c368
----------
Ultravate®
(halobetasol propionate) Cream, 0.05%
(halobetasol propionate) Ointment, 0.05%
For Dermatological Use Only. Not for Ophthalmic Use.
Rx only
DESCRIPTION
Ultravate® contains halobetasol propionate, a synthetic corticosteroid for topical dermatological use. The corticosteroids constitute a class of primarily synthetic steroids used topically as an anti-inflammatory and antipruritic agent.
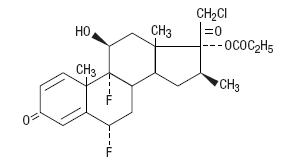
Chemically halobetasol propionate is 21-chloro-6α, 9-difluoro-11β, 17-dihydroxy-16β-methylpregna-1, 4-diene-3-20-dione, 17-propionate, C25H31ClF2O5. It has the following structural formula:
Halobetasol propionate has the molecular weight of 485. It is a white crystalline powder insoluble in water.
Each gram of Ultravate Cream contains 0.5 mg/g of halobetasol propionate in a cream base of cetyl alcohol, glycerin, isopropyl isostearate, isopropyl palmitate, steareth-21, diazolidinyl urea, methylchloroisothiazolinone, (and) methylisothiazolinone and water.
Each gram of Ultravate Ointment contains 0.5 mg/g of halobetasol propionate in a base of aluminum stearate, beeswax, pentaerythritol cocoate, petrolatum, propylene glycol, sorbitan sesquioleate, and stearyl citrate.
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, halobetasol propionate has anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of the anti-inflammatory activity of the topical corticosteroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
Human and animal studies indicate that less than 6% of the applied dose of halobetasol propionate enters the circulation within 96 hours following topical administration of Ultravate.
Studies performed with Ultravate indicate that it is in the super-high range of potency as compared with other topical corticosteroids.
INDICATIONS AND USAGE
Ultravate is a super-high potency corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. Treatment beyond two consecutive weeks is not recommended, and the total dosage should not exceed 50 g/week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Use in children under 12 years of age is not recommended.
As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
CONTRAINDICATIONS
Ultravate is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
PRECAUTIONS
General
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing’s syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
Patients applying a topical steroid to a large surface area or to areas under occlusion should be evaluated periodically for evidence of HPA axis suppression. This may be done by using the ACTH stimulation, A.M. plasma cortisol, and urinary free-cortisol tests. Patients receiving super potent corticosteroids should not be treated for more than 2 weeks at a time and only small areas should be treated at any one time due to the increased risk of HPA suppression.
Ultravate produced HPA axis suppression when used in divided doses at 7 grams per day for one week in patients with psoriasis. These effects were reversible upon discontinuation of treatment.
If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid. Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios (see PRECAUTIONS: Pediatric Use).
If irritation develops, Ultravate should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of Ultravate should be discontinued until the infection has been adequately controlled.
Ultravate should not be used in the treatment of rosacea or perioral dermatitis, and it should not be used on the face, groin, or in the axillae.
Information for Patients
Patients using topical corticosteroids should receive the following information and instructions:
- 1. The medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- 2. The medication should not be used for any disorder other than that for which it was prescribed.
- 3. The treated skin area should not be bandaged, otherwise covered or wrapped, so as to be occlusive unless directed by the physician.
- 4. Patients should report to their physician any signs of local adverse reactions.
Laboratory Tests
The following tests may be helpful in evaluating patients for HPA axis suppression: ACTH-stimulation test; A.M. plasma cortisol test; Urinary free-cortisol test.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of halobetasol propionate. Positive mutagenicity effects were observed in two genotoxicity assays. Halobetasol propionate was positive in a Chinese hamster micronucleus test, and in a mouse lymphoma gene mutation assay in vitro.
Studies in the rat following oral administration at dose levels up to 50 mcg/kg/day indicated no impairment of fertility or general reproductive performance.
In other genotoxicity testing, halobetasol propionate was not found to be genotoxic in the Ames/Salmonella assay, in the sister chromatid exchange test in somatic cells of the Chinese hamster, in chromosome aberration studies of germinal and somatic cells of rodents, and in a mammalian spot test to determine point mutations.
Pregnancy
Teratogenic effects: Pregnancy Category C
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Halobetasol propionate has been shown to be teratogenic in SPF rats and chinchilla-type rabbits when given systemically during gestation at doses of 0.04 to 0.1 mg/kg in rats and 0.01 mg/kg in rabbits. These doses are approximately 13, 33 and 3 times, respectively, the human topical dose of Ultravate. Halobetasol propionate was embryotoxic in rabbits but not in rats.
Cleft palate was observed in both rats and rabbits. Omphalocele was seen in rats, but not in rabbits.
There are no adequate and well-controlled studies of the teratogenic potential of halobetasol propionate in pregnant women. Ultravate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Ultravate is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of Ultravate in pediatric patients have not been established and use in pediatric patients under 12 is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Geriatric Use
Of approximately 400 patients treated with Ultravate® Cream in clinical studies, 25% were 61 years and over and 6% were 71 years and over and 850 patients treated with Ultravate® Ointment in clinical studies, 21% were 61 years and over and 6% were 71 years and over. No overall differences in safety or effectiveness were observed between these patients and younger patients; and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
ADVERSE REACTIONS
In controlled clinical trials, the most frequent adverse events reported for Ultravate Cream included stinging, burning or itching in 4.4% of the patients. Less frequently reported adverse reactions were dry skin, erythema, skin atrophy, leukoderma, vesicles and rash.
In controlled clinical trials, the most frequent adverse events reported for Ultravate Ointment included stinging or burning in 1.6% of the patients. Less frequently reported adverse reactions were pustulation, erythema, skin atrophy, leukoderma, acne, itching, secondary infection, telangiectasia, urticaria, dry skin, miliaria, paresthesia, and rash.
The following additional local adverse reactions are reported infrequently with topical corticosteroids, and they may occur more frequently with high potency corticosteroids, such as Ultravate. These reactions are listed in an approximate decreasing order of occurrence: folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, striae and miliaria.
OVERDOSAGE
Topically applied Ultravate can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
DOSAGE AND ADMINISTRATION
Apply a thin layer of Ultravate Cream or Ointment to the affected skin once or twice daily, as directed by your physician, and rub in gently and completely.
Ultravate (halobetasol propionate) is a super-high potency topical corticosteroid; therefore, treatment should be limited to two weeks, and amounts greater than 50 g/wk should not be used. As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary.
Ultravate should not be used with occlusive dressings.
HOW SUPPLIED
Ultravate® (halobetasol propionate) Cream, 0.05% is a smooth white cream having a characteristic odor and supplied in the following tube size:
50 g (NDC: 10631-103-50)
2 x 50 g (NDC: 10631-103-04)
Ultravate® (halobetasol propionate) Ointment, 0.05% is an off-white, smooth ointment with characteristic odor; essentially free from foreign matter and supplied in the following tube size:
50 g (NDC: 10631-102-50)
2 x 50 g (NDC: 10631-102-04)
| ULTRAVATE
halobetasol propionate cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ULTRAVATE
halobetasol propionate ointment |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Contract Pharmaceuticals Limited | 248761249 | MANUFACTURE(10631-103, 10631-102) , PACK(10631-103, 10631-102) | |