VANACOF DMX- dextromethorphan hydrobromide liquid
Vanacof DMX by
Drug Labeling and Warnings
Vanacof DMX by is a Otc medication manufactured, distributed, or labeled by GM Pharmaceuticals, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
■ temporarily relieves:
■ cough due to minor throat and bronchial irritation as
may occur with the common cold or inhaled irritants
■ the intensity of coughing
■ nasal congestion due to a cold
■ the impulse to cough to help you get to sleep
■ helps loosen phlegm (mucus) and thin bronchial
secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
-
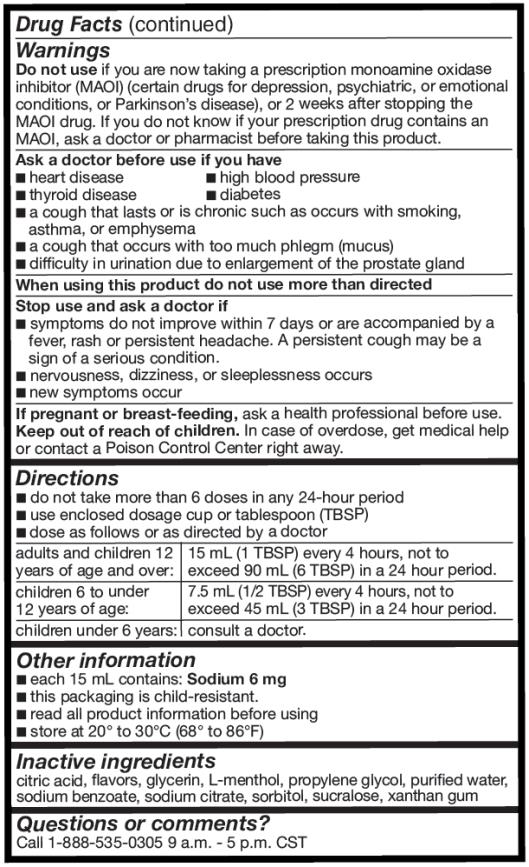
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
■ heart disease ■ high blood pressure
■ thyroid disease ■ diabetes
■ a cough that lasts or is chronic such as occurs with smoking, asthma, or emphysema
■ a cough that occurs with too much phlegm (mucus)
■ difficulty in urination due to enlargement of the prostate gland
-
Directions
■ do not take more than 6 doses in any 24-hour period
■ use enclosed dosage cup or tablespoon (TBSP)
■ dose as follows or as directed by a doctor
adults and children 12 years of age and over: 15 mL (1 TBSP) every 4 hours, not to exceed 90 mL (6 TBSP) in a 24 hour period. children 6 to under 12 years of age: 7.5 mL (1/2 TBSP) every 4 hours, not to exceed 45 mL (3 TBSP) in a 24 hour period. children under 6 years of age: Consult a doctor. - Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VANACOF DMX
dextromethorphan hydrobromide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58809-850 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 18 mg in 15 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 396 mg in 15 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 15 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) LEVOMENTHOL (UNII: BZ1R15MTK7) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor RASPBERRY, MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58809-850-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/12/2019 Labeler - GM Pharmaceuticals, INC (793000860)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.