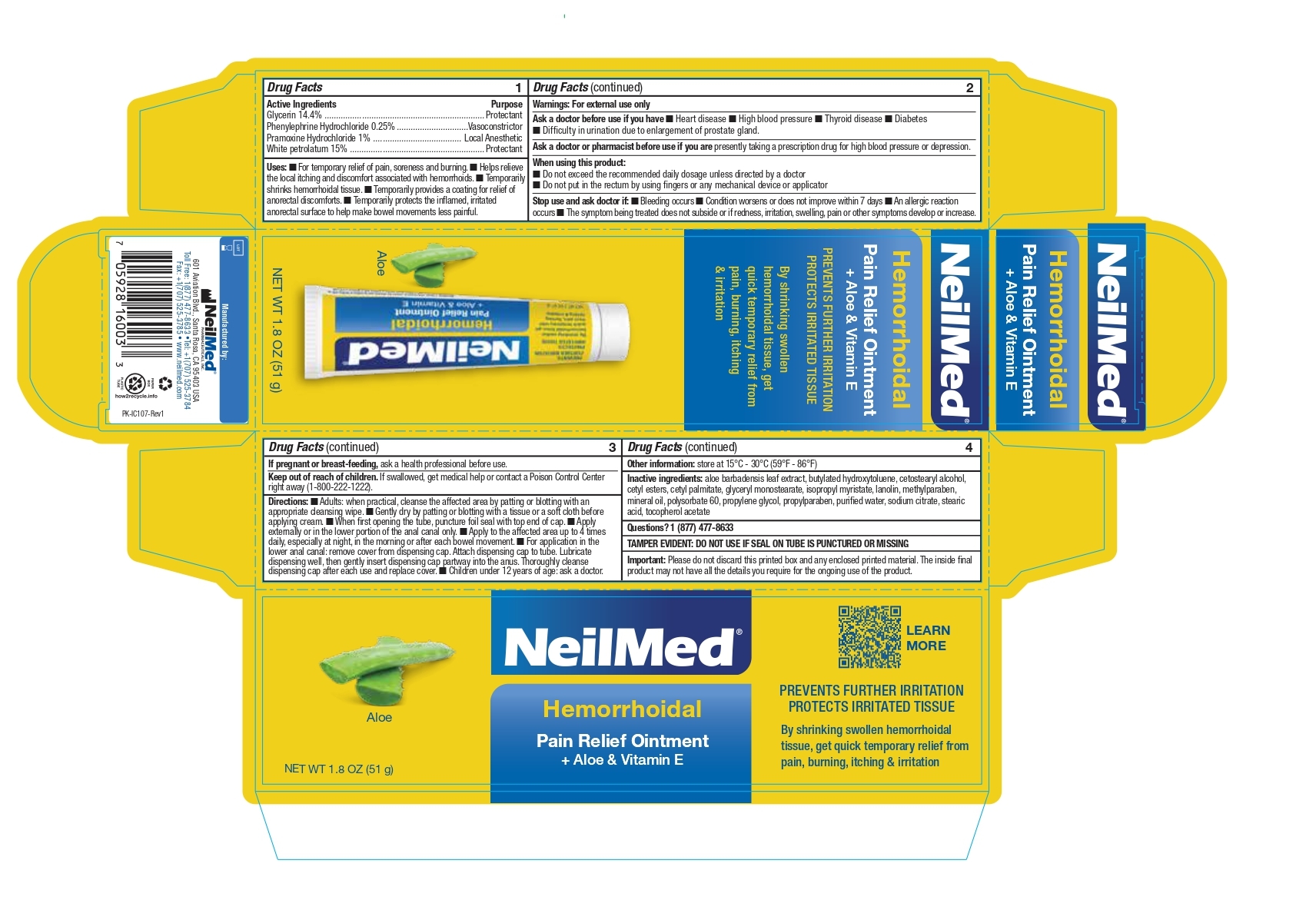
NeilMed Hemorrhoidal Pain Relief Ointment with Aloe and Vitamin E 51g
Hemorrhoidal Pain Relief by
Drug Labeling and Warnings
Hemorrhoidal Pain Relief by is a Otc medication manufactured, distributed, or labeled by NeilMed Pharmaceuticals Inc., NeilMed pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEMORRHOIDAL PAIN RELIEF- hemorrhoidal pain relief with aloe and vitamin e ointment
NeilMed Pharmaceuticals Inc.
----------
NeilMed Hemorrhoidal Pain Relief Ointment with Aloe and Vitamin E 51g
Active Ingredients
Active Ingredients
Glycerin 14.4%
Phenylephrine Hydrochloride 0.25%
Pramoxine Hydrochloride 1%
White petrolatum 15%
Drug Facts
Active Ingredients Purpose
Glycerin 14.4% ..................................................................... Protectant
Phenylephrine Hydrochloride 0.25% ...............................Vasoconstrictor
Pramoxine Hydrochloride 1% ...................................... Local Anesthetic
White petrolatum 15% .......................................................... Protectant
Drug Facts :
Uses
For temporary relief of pain, soreness and burning.
Helps relieve the local itching and discomfort associated with hemorrhoids.
Temporarily shrinks hemorrhoidal tissue.
Temporarily provides a coating for relief of anorectal discomforts.
Temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful.
Ask a doctor before use if you have
Heart disease High blood pressure Thyroid disease DiabetesDifficulty in urination due to enlargement of prostate gland.
Ask a doctor or pharmacist before use
Ask a doctor or pharmacist before use if you are presently taking a prescription drug for high blood pressure or depression.
When using this product:
Do not exceed the recommended daily dosage unless directed by a doctor
Do not put in the rectum by using fingers or any mechanical device or applicator
Stop use and ask doctor if:
Bleeding occurs Condition worsens or does not improve within 7 days An allergic reaction occurs The symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222).
Directions:
Adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying cream. When first opening the tube, puncture foil seal with top end of cap. Apply externally or in the lower portion of the anal canal only. Apply to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement. For application in the lower anal canal: remove cover from dispensing cap. Attach dispensing cap to tube. Lubricate dispensing well, then gently insert dispensing cap partway into the anus. Thoroughly cleanse
dispensing cap after each use and replace cover. Children under 12 years of age: ask a doctor.
Inactive ingredients:
aloe barbadensis leaf extract, butylated hydroxytoluene, cetostearyl alcohol, cetyl esters, cetyl palmitate, glyceryl monostearate, isopropyl myristate, lanolin, methylparaben, mineral oil, polysorbate 60, propylene glycol, propylparaben, purified water, sodium citrate, stearic acid, tocopherol acetate
| HEMORRHOIDAL PAIN RELIEF
hemorrhoidal pain relief with aloe and vitamin e ointment |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - NeilMed Pharmaceuticals Inc. (799295915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| NeilMed pharmaceuticals Inc. | 799295915 | manufacture(13709-320) | |