Anti-Bacterial Hand by Bath & Body Works, Inc. / Knowlton Development Corporation
Anti-Bacterial Hand by
Drug Labeling and Warnings
Anti-Bacterial Hand by is a Otc medication manufactured, distributed, or labeled by Bath & Body Works, Inc., Knowlton Development Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
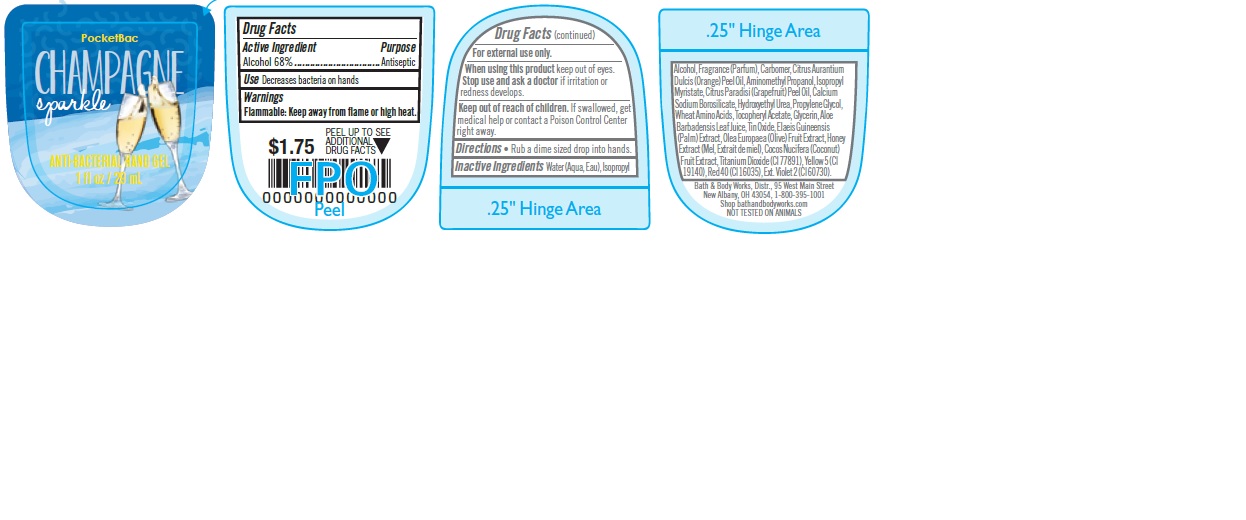
ANTI-BACTERIAL HAND CHAMPAGNE SPARKLE- alcohol gel
Bath & Body Works, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENTS
Water (Aqua, Eau), Isopropyl Alcohol, Fragrance (Parfum), Carbomer, Citrus Aurantium Dulcis (Orange) Peel Oil, Aminomethyl Propanol, Isopropyl Myristate, Citrus Paradisi (Grapefruit) Peel Oil, Calcium Sodium Borosilicate, Hydroxyethyl Urea, Propylene Glycol, Wheat Amino Acids, Tocopheryl Acetate, Glycerin, Aloe Barbadensis Leaf Juice, Tin Oxide, Elaeis Guineensis (Palm) Extract, Olea Europaea (Olive) Fruit Extract, Honey Extract (Mel, Extrait de miel), Cocos Nucifera (Coconut) Fruit Extract, Titanium Dioxide (CI 77891), Yellow 5 (CI 19140), Red 40 (CI 16035), Ext. Violet 2 (CI 60730).
| ANTI-BACTERIAL HAND
CHAMPAGNE SPARKLE
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bath & Body Works, Inc. (878952845) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Knowlton Development Corporation | 968939913 | manufacture(62670-5591) | |