solutions plus ® Secure Gel Hand Sanitizer
Secure Gel Hand Sanitizer by
Drug Labeling and Warnings
Secure Gel Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by American Cleaning Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SECURE GEL HAND SANITIZER- isopropyl alcohol gel
American Cleaning Solutions
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
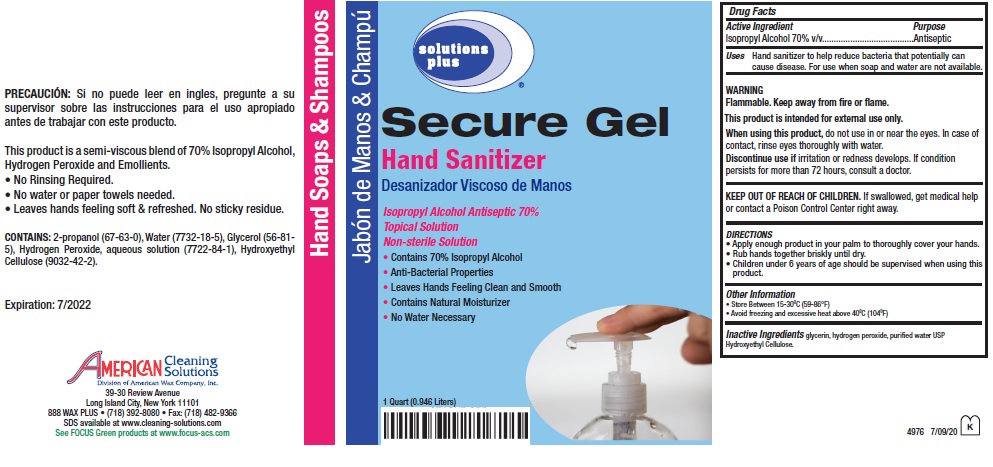
solutions plus ® Secure Gel Hand Sanitizer
Uses Hand sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and
water are not available.
WARNING
Flammable. Keep away from fire or flame.
This product is intended for external use only.
When using this product, do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
Discontinue use if irritation or redness develops. If condition persists for more than 72 hours, consult a doctor.
DIRECTIONS
Apply enough product in your palm to thoroughly cover your hands.
Rub hands together briskly until dry.
Children under 6 years of age should be supervised when using this product.
Other Information
Store Between 15-30°C (59-86°F)
Avoid freezing and excessive heat above 40°C (104°F)
Desanizador Viscoso de Manos
Topical Solution
Non-sterile Solution
Contains 70% Isopropyl Alcohol
Anti-Bacterial Properties
Leaves Hands Feeling Clean and Smooth
Contains Natural Moisturizer
No Water Necessary
Hand Soaps & Shampoos
This product is a semi-viscous blend of 70% Isopropyl Alcohol, Hydrogen Peroxide and Emollients.
No Rinsing Required.
No water or paper towels needed.
Leaves hands feeling soft & refreshed. No sticky residue.
CONTAINS: 2-propanol (67-63-0), Water (7732-18-5), Glycerol (56-81-5), Hydrogen Peroxide,
aqueous solution (7722-84-1), Hydroxyethyl Cellulose(9032-42-2).
Expiration: 7/2022
AMERICAN Cleaning Solutions
Division of American Wax Company. Inc
39-30 Review Avenue
Long Island City, New York 11101
888 WAX PLUS (718) 392-8080 Fax: (718) 482-9366
SDS available at www.cleaning-solutions.com
See FOCUS Green products at www.focus-acs.com
| SECURE GEL HAND SANITIZER
isopropyl alcohol gel |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - American Cleaning Solutions (001321017) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| American Cleaning Solutions | 001321017 | manufacture(49922-976) | |