DRY-CID- dry concentrate for hemodialysis powder, for solution
DRY-CID by
Drug Labeling and Warnings
DRY-CID by is a Otc medication manufactured, distributed, or labeled by Aqua Medica, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
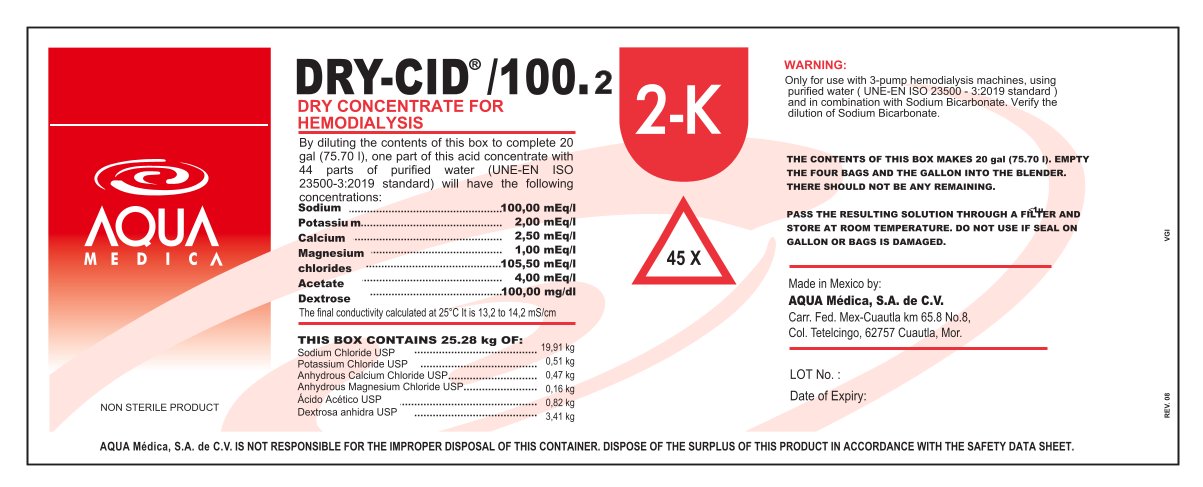
By diluting the contents of this box to complete 20
gal (75.70 l), one part of this acid concentrate with
44 parts of purified water (UNE-EN ISO
23500-3:2019 standard) will have the following
concentrations:
Sodium
Potassiu m
Calcium
Magnesium
chlorides
Acetate
Dextrose
The final conductivity calculated at 25°C It is 13,2 to 14,2 mS/cm
NON STERILE PRODUCT
100,00 mEq/l
2,00 mEq/l
2,50 mEq/l
1,00 mEq/l
105,50 mEq/l
4,00 mEq/l
100,00 mg/dl - ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DRY-CID
dry concentrate for hemodialysis powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 81943-601 Route of Administration HEMODIALYSIS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM CHLORATE (UNII: H35KS68EE7) (CHLORATE ION - UNII:08Z8093742) POTASSIUM CHLORATE 0.51 kg in 100 kg Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 19.91 kg in 100 kg CALCIUM CHLORIDE ANHYDROUS (UNII: OFM21057LP) 0.47 kg in 100 kg MAGNESIUM CHLORIDE ANHYDROUS (UNII: 59XN63C8VM) 0.16 kg in 100 kg ACETIC ACID (UNII: Q40Q9N063P) 0.82 kg in 100 kg ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) 3.41 kg in 100 kg WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 81943-601-01 25.28 kg in 1 BOX; Type 0: Not a Combination Product 08/30/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/30/2023 Labeler - Aqua Medica, S.A. de C.V. (589696442) Establishment Name Address ID/FEI Business Operations Aqua Medica, S.A. de C.V. 589696442 manufacture(81943-601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 81943-601-01
81943-601-01