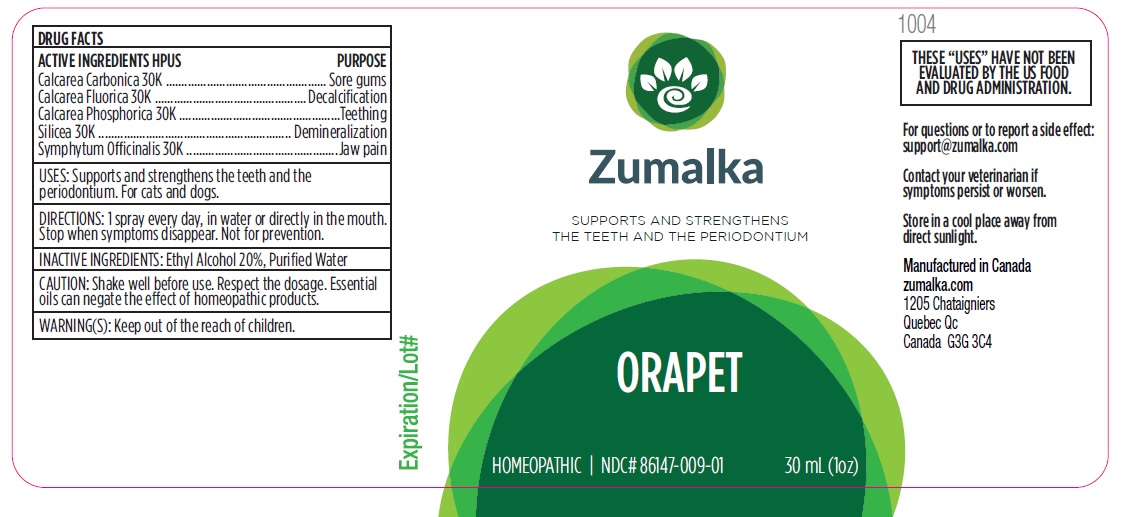
ORAPET by Groupe Cyrenne Inc.
ORAPET by
Drug Labeling and Warnings
ORAPET by is a Homeopathic medication manufactured, distributed, or labeled by Groupe Cyrenne Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ORAPET- oyster shell calcium carbonate, crude, comfrey root, calcium fluoride, tribasic calcium phosphate, tribasic calcium phosphate, silicon dioxide liquid
Groupe Cyrenne Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Active ingredient Purpose
Calcarea Carbonica 30k Sore gums
Calcarea Fluorica 30k Decalcification
Calcarea Phosphorica 30k Teething
Silicea 30k Demineralization
Symphytum Officinalis 30k Jaw pain
Direction
1 spray every day, in water or directly in the mouth. Stop when symptoms disappear. Not for prevention.
| ORAPET
oyster shell calcium carbonate, crude, comfrey root, calcium fluoride, tribasic calcium phosphate, tribasic calcium phosphate, silicon dioxide liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Groupe Cyrenne Inc. (208482650) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Laboratoire Schmidt-Nagel Inc | 203869883 | api manufacture | |
Trademark Results [ORAPET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ORAPET 85964760 not registered Dead/Abandoned |
ESPREE ANIMAL PRODUCTS, INC. 2013-06-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.