PURESKIN FUNGAL NAIL RENEWAL by Stellans Inc. 83565-003 Completed
PURESKIN FUNGAL NAIL RENEWAL by
Drug Labeling and Warnings
PURESKIN FUNGAL NAIL RENEWAL by is a Otc medication manufactured, distributed, or labeled by Stellans Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
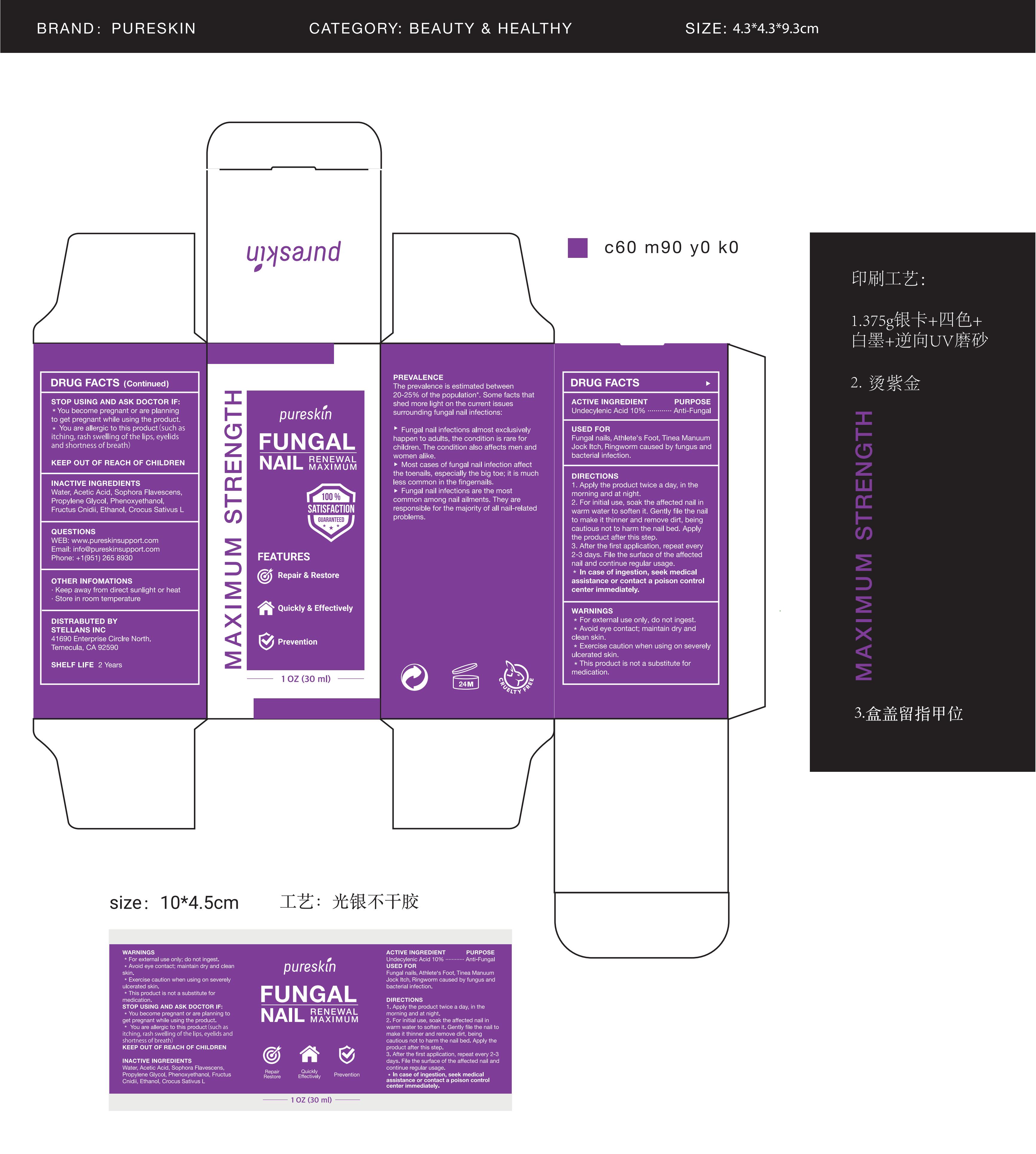
PURESKIN FUNGAL NAIL RENEWAL- fungal nail renewal liquid
Stellans Inc.
----------
83565-003 Completed
Use
Fungal nails
Athlete's Foot
Tinea Manuum
Jock Itch
Ringworm caused by fungus and bacterial infection.
Warnings
For external use only; do not ingest.
Avoid eye contact; maintain dry and clean skin.
Exercise caution when using on severely ulcerated skin.
This product is not a substitute for medication.
When Using
Stop Use And See A Doctor If
You become pregnant or are planning to get pregnant while using the product.
You are allergic to this product (such as itching, rash swelling of the lips, eyelids, and shortness of breath).
Ask Doctor
If you are allergic to this product. If symptoms persist for over 7 days or worsen, consult your physician. If you are pregnant or breast-feeding - You become pregnant or are planning to ...
Keep Oot Of Reach Of Children
Keep out of reach of children.In case of ingestion, call Poison Control Center hotline immediately or seek medical help.
Directions
1. Apply the product twice a day, in the morning and at night.
2. For initial use, soak the affected nail in warm water to soften it. Gently file the nail to make it thinner and remove dirt, being cautious not to harm the nail bed. Apply the product after this step.
3. After the first application, repeat every 2-3 days. File the surface of the affected nail and continue regular usage.
In case of ingestion, seek medical assistance or contact a poison control center immediately.
| PURESKIN FUNGAL NAIL RENEWAL
fungal nail renewal liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Stellans Inc. (111157321) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Stellans Inc. | 111157321 | label(83565-003) , manufacture(83565-003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.