NATACHEW- .beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, folic acid, cyanocobalamin, iron, niacinamide tablet, chewable
NataChew by
Drug Labeling and Warnings
NataChew by is a Prescription medication manufactured, distributed, or labeled by Eckson Labs, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
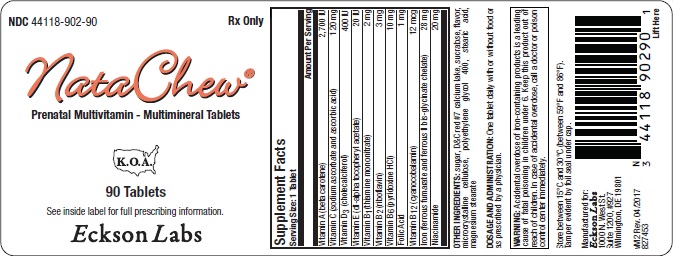
Supplement Facts
Serving Size: 1 TabletAmount Per Serving Vitamin A (beta carotene) 2,700 IU Vitamin C (sodium ascorbate and ascorbic acid) 120 mg Vitamin D 3 (cholecalciferol) 400 IU Vitamin E (dl-alpha tocopheryl acetate) 20 IU Vitamin B 1 (thiamine mononitrate) 2 mg Vitamin B 2 (riboflavin) 3 mg Vitamin B 6 (pyridoxine HCl) 10 mg Folic Acid 1 mg Vitamin B 12 (cyanocobalamin) 12 mcg Iron (ferrous fumarate and ferrous II bis-glycinate chelate) 28 mg Niacinamide 20 mg OTHER INGREDIENTS: sugar, D&C red #7 calcium lake, sucralose, flavor, microcrystalline cellulose, polyethylene glycol 400, stearic acid, magnesium stearate
-
INDICATIONS AND USAGE:
NataChew ® is a prescription pre/post-natal multivitamin, multimineral indicated for use in improving the nutritional status of women throughout pregnancy, as well as in the post-natal period for both lactating and non-lactating mothers. This product is also useful in improving the nutritional status of women prior to conception.
- CONTRAINDICATIONS:
-
WARNINGS AND PRECAUTIONS:
Folic acid is improper therapy in the treatment of pernicious anemia in that hematological remission can occur while neurological manifestations remain progressive. While prescribing this product for pregnant women, nursing mothers, or for women prior to conception, their medical condition and use of other drugs, herbs, and/or supplements should be considered.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
- ADVERSE REACTIONS:
- DOSAGE AND ADMINISTRATION:
-
HOW SUPPLIED:
NataChew ® is supplied as a red tablet, imprinted “902”, in child-resistant bottles of 90 tablets, NDC: 44118-902-90.
Store between 15°C and 30°C (between 59°F and 86°F). Tamper evident by foil seal under cap.
Manufactured for:
Eckson Labs, LLC
1000 N. West St., Suite 1200, #927
Wilmington, DE 19801
vM2 Rev. 04/2017 827453 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATACHEW
.beta.-carotene, ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, d-, thiamine mononitrate, riboflavin, pyridoxine hydrochloride, folic acid, cyanocobalamin, iron, niacinamide tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 44118-902 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .BETA.-CAROTENE (UNII: 01YAE03M7J) (.BETA.-CAROTENE - UNII:01YAE03M7J) .BETA.-CAROTENE 2700 [iU] ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 20 [iU] THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE MONONITRATE 2 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 28 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) D&C RED NO. 7 (UNII: ECW0LZ41X8) SUCRALOSE (UNII: 96K6UQ3ZD4) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color red Score no score Shape ROUND Size 13mm Flavor CHERRY Imprint Code 902 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44118-902-90 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/15/2012 2 NDC: 44118-902-05 9 in 1 CARTON 10/15/2012 2 5 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/15/2012 Labeler - Eckson Labs, LLC (078435242)
Trademark Results [NataChew]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NATACHEW 77839662 3901675 Live/Registered |
Mission Pharmacal Company 2009-10-01 |
 NATACHEW 75748949 not registered Dead/Abandoned |
MISSION PHARMACAL COMPANY 1999-06-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.