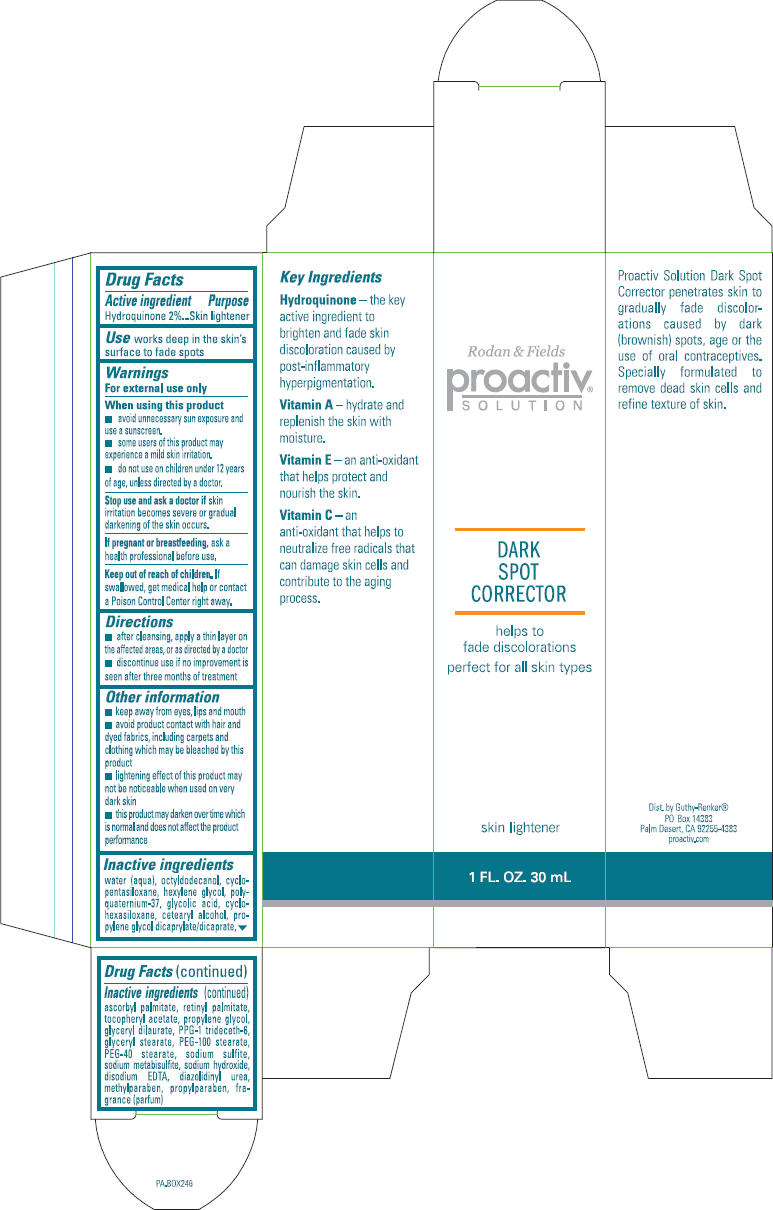
PROACTIV SOLUTION DARK SPOT CORRECTOR- hydroquinone lotion
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Hydroquinone 2%
Use
works deep in the ski's surface to fade spots
Warnings
For external use only
- avoid unnecessary sun exposure and use a sunscreen.
- some users of this product may experience a mild skin irritation.
- do not use on children under 12 years of age, unless directed by a doctor.
Stop use and ask a doctor if skin irritation becomes severe or gradual darkening of the skin occurs.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- After cleansing, apply a thin layer on the affected areas, or as directed by a doctor
- discontinue use if no improvement is seen after three months of treatment
Other information
- keep away from eyes, lips and mouth
- avoid product contact with hair and dyed fabrics, including carpets and clothing which may be bleached by this product
- lightening effect of this product may not be noticeable when used on very dark skin
- this product may darken over time which is normal and does not affect the product performance
Inactive ingredients
Water, octyldodecanol, cyclopentasiloxane, hexylene glycol, polyquaternium-37, glycolic acid, cyclohexasiloxane, cetearyl alcohol, propylene glycol dicaprylate/dicaprate, ascorbyl palmitate, retinyl palmitate, tocopheryl acetate, propylene glycol, glyceryl dilaurate, PPG-1 trideceth-6, glyceryl stearate, PEG-100 stearate, PEG-40 stearate, sodium sulfite, sodium metabisulfite, sodium hydroxide, disodium EDTA, diazolidinyl urea, methylparaben, propylparaben, fragrance
Questions or comments?
Within US 1-800-490-4134
Dist. by Guthy-Renker®
PO Box 14383
Palm Desert, CA 14383
PRINCIPAL DISPLAY PANEL - 30 mL Tube Box
Rodan & Fields
proactiv®
SOLUTION
DARK
SPOT
CORRECTOR
helps to
fade discolorations
perfect for all skin types
skin lightener
1 FL. OZ. 30 mL