ALLERGY RELIEF- fexofenadine hcl tablet, coated
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by P & L Development, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
allergy relief
Fexofenadine HCL USP 180 mg tablets
antihistamine
- for indoor and outdoor allergies
- non-drowsy
caplets**
(**capsule-shaped tablets)
*Compare to the active ingredient in Allegra® Allergy 24 Hour
*This product is not manufactured or distributed by Chattem Inc., distributor of Allegra® Allergy 24 hour.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: PL Developments
200 Hicks Street Westbury, NY 11590
- Product Label
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
fexofenadine hcl tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59726-564 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color PINK Score no score Shape CAPSULE Size 18mm Flavor Imprint Code J;44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59726-564-15 1 in 1 BOX 10/01/2019 1 150 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204097 10/01/2019 Labeler - P & L Development, LLC (800014821)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
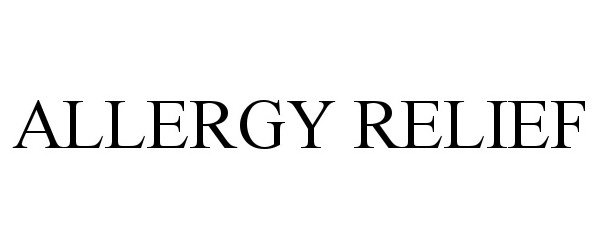 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
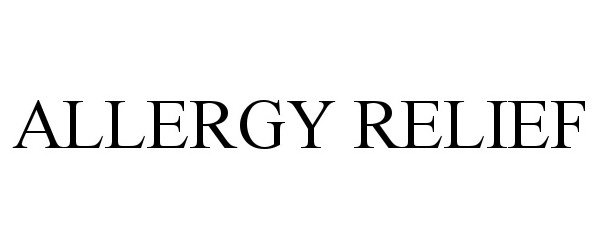 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
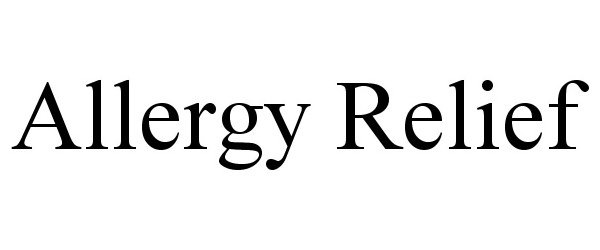 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
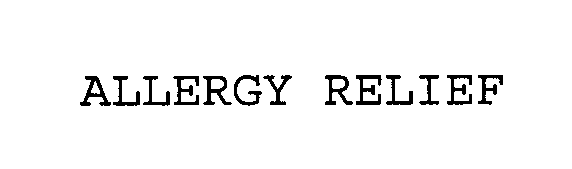 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
