FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS) EMERGENCY USE AUTHORIZATION (EUA)
Moderna COVID-19 Vaccine, Bivalent by
Drug Labeling and Warnings
Moderna COVID-19 Vaccine, Bivalent by is a Other medication manufactured, distributed, or labeled by Moderna US, Inc., ModernaTX, Inc., Catalent Indiana, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MODERNA COVID-19 VACCINE, BIVALENT- moderna covid-19 vaccine, bivalent injection, suspension
Moderna US, Inc.
----------
FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS)
EMERGENCY USE AUTHORIZATION (EUA)
|
|
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to permit the emergency use of the unapproved product, Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), for active immunization to prevent COVID-19 in individuals 6 months of age and older.
Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) is hereafter referred to as Moderna COVID-19 Vaccine, Bivalent.
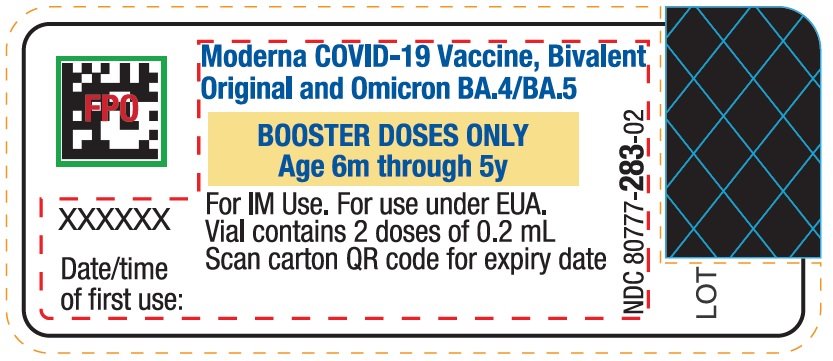
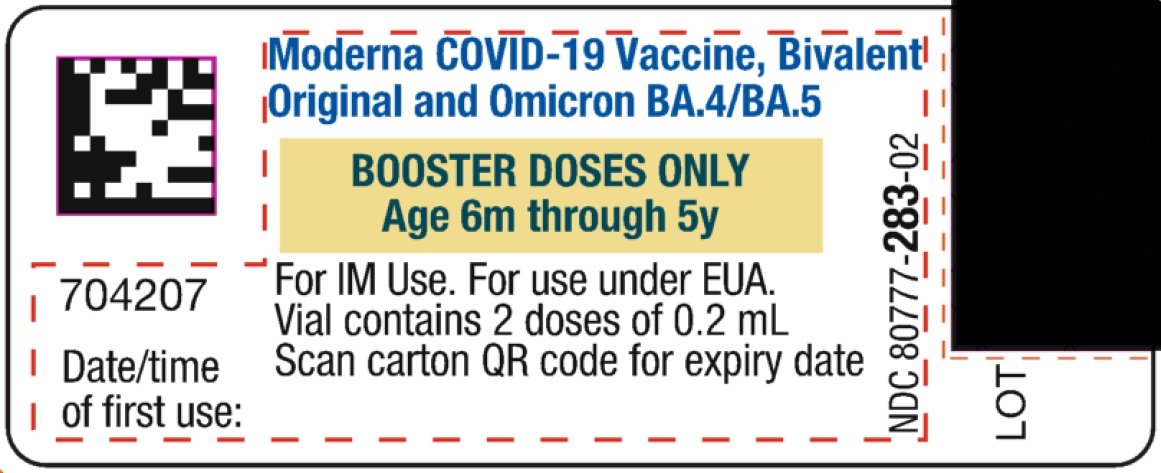
This Fact Sheet pertains only to Moderna COVID-19 Vaccine, Bivalent supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box (see image below) which is authorized for use in individuals 6 months through 5 years of age as a single booster dose administered at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine.
Moderna COVID-19 Vaccine supplied in multiple-dose vials with a dark pink cap and a label with a yellow box intended for use in individuals 6 months through 5 years of age should not be used in individuals 6 years of age and older because of the potential for vaccine administration errors, including dosing errors.1
SUMMARY OF INSTRUCTIONS FOR COVID-19 VACCINATION PROVIDERS
Vaccination providers enrolled in the federal COVID-19 Vaccination Program must report all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and cases of COVID-19 that result in hospitalization or death following administration of Moderna COVID-19 Vaccine, Bivalent. See “MANDATORY REQUIREMENTS FOR MODERNA COVID-19 VACCINE, BIVALENT, ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION” for reporting requirements.
Moderna COVID-19 Vaccine, Bivalent is a suspension for intramuscular injection.
Moderna COVID-19 Vaccine, Bivalent, supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box, is administered as a single booster dose at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine.
For individuals 6 months through 5 years of age, a single booster dose is 0.2 mL.
See this Fact Sheet for instructions for preparation and administration. This Fact Sheet may have been updated. For the most recent Fact Sheet, please see www.modernatx.com/covid19vaccine-eua.
For information on clinical trials that are testing the use of the Moderna COVID-19 Vaccine, Bivalent for active immunization against COVID-19, please see www.clinicaltrials.gov.
DESCRIPTION OF COVID-19
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the novel coronavirus, SARS-CoV-2, that appeared in late 2019. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have reported a wide range of symptoms, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle and body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
DOSAGE AND ADMINISTRATION
Storage and Handling
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Frozen Storage
Store frozen between -50°C to -15°C (-58°F to 5°F).
Storage after Thawing
-
Storage at 2°C to 8°C (36°F to 46°F):
- o Vials may be stored refrigerated between 2°C to 8°C (36°F to 46°F) for up to 30 days prior to first use, provided the expiration date is not exceeded.
- o Vials should be discarded 8 hours after the first puncture.
-
Storage at 8°C to 25°C (46°F to 77°F):
- o Vials may be stored between 8°C to 25°C (46°F to 77°F) for a total of 24 hours.
- o Vials should be discarded 8 hours after the first puncture.
- o Total storage at 8°C to 25°C (46°F to 77°F) must not exceed 24 hours.
Do not refreeze once thawed.
Thawed vials can be handled in room light conditions.
Transportation of Thawed Vials at 2°C to 8°C (36°F to 46°F)
If transport at -50°C to -15°C (-58°F to 5°F) is not feasible, available data support transportation of one or more thawed vials for up to 12 hours at 2°C to 8°C (36°F to 46°F) when shipped using shipping containers which have been qualified to maintain 2°C to 8°C (36°F to 46°F) and under routine road and air transport conditions with shaking and vibration minimized. Once thawed and transported at 2°C to 8°C (36°F to 46°F), vials should not be refrozen and should be stored at 2°C to 8°C (36°F to 46°F) until use.
Dose and Schedule
Moderna COVID-19 Vaccine, Bivalent, supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box, is administered as a single booster dose at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine.
For individuals 6 months through 5 years of age, a single booster dose is 0.2 mL.
Preparation for Administration
- Moderna COVID-19 Vaccine, Bivalent multiple-dose vial with a dark pink cap and a label with a yellow box is supplied as a frozen suspension that does not contain a preservative and must be thawed prior to administration.
- Verify that the vial has a dark pink cap and a label with a yellow box and that the label states Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5).
- Thaw each vial before use following the instructions below.
- Thawing Instructions for Moderna COVID-19 Vaccine, Bivalent Multiple-Dose Vials with Dark Pink Caps and Labels with a Yellow Box
|
Thaw in Refrigerator |
Thaw at Room Temperature |
|
Thaw between 2°C to 8°C (36°F to 46°F) for 45 minutes. Let each vial stand at room temperature for 15 minutes before administering. |
Alternatively, thaw between 15°C to 25°C (59°F to 77°F) for 15 minutes. |
- After thawing, do not refreeze.
- Swirl vial gently after thawing and between each withdrawal. Do not shake. Do not dilute the vaccine.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Moderna COVID-19 Vaccine, Bivalent is a white to off-white suspension. It may contain white or translucent product-related particulates. Do not administer if vaccine is discolored or contains other particulate matter.
- A booster dose for individuals 6 months through 5 years of age is 0.2 mL.
- Each multiple-dose vial contains 2 booster doses of 0.2 mL.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.2 mL, discard the vial and contents. Do not pool excess vaccine from multiple vials.
- After the first booster dose has been withdrawn, the vial should be held between 2°C to 25°C (36°F to 77°F). Record the date and time of first use on the Moderna COVID-19 Vaccine, Bivalent vial label. Discard vial after 8 hours. Do not refreeze.
CONTRAINDICATION
Do not administer Moderna COVID-19 Vaccine, Bivalent to individuals with a known history of a severe allergic reaction (e.g., anaphylaxis) to any component of Moderna COVID-19 Vaccine, Bivalent (see Full EUA Prescribing Information).
WARNINGS
Management of Acute Allergic Reactions
Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Moderna COVID-19 Vaccine, Bivalent.
Monitor Moderna COVID-19 Vaccine, Bivalent recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
Myocarditis and Pericarditis
Postmarketing safety data with Moderna COVID-19 Vaccine are relevant to Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
Postmarketing data with authorized or approved monovalent mRNA COVID-19 vaccines demonstrate increased risks of myocarditis and pericarditis, particularly within the first week following receipt of the second primary series dose or first booster dose, with most booster doses likely administered at least 5 months after completing primary vaccination. For the Moderna COVID-19 Vaccine, the observed risk is highest in males 18 through 24 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae.
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
Altered Immunocompetence
Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to Moderna COVID-19 Vaccine, Bivalent.
Limitations of Vaccine Effectiveness
Moderna COVID-19 Vaccine, Bivalent may not protect all vaccine recipients.
ADVERSE REACTIONS
The safety of a booster dose of Moderna COVID-19 Vaccine, Bivalent for use in individuals 6 months through 5 years of age is based on:
- safety data from a clinical study which evaluated a booster dose of Moderna’s bivalent COVID-19 vaccine (Original and Omicron BA.1), not authorized or approved in the U.S., hereafter referred to as bivalent vaccine (Original and Omicron BA.1),
- safety data from clinical trials which evaluated primary and booster vaccination with Moderna COVID-19 Vaccine,2 and
- postmarketing safety data with Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent
The safety data accrued with the bivalent vaccine (Original and Omicron BA.1) and with Moderna COVID-19 Vaccine are relevant to Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
Adverse Reactions in Clinical Trials
Adverse reactions in individuals 17 months through 36 months of age following administration of a single booster dose of Moderna COVID-19 Vaccine included irritability/crying, pain at the injection site, sleepiness, loss of appetite, erythema at the injection site, swelling at the injection site, fever, axillary (or groin) swelling/tenderness. (See Full EUA Prescribing Information)
Adverse reactions in individuals 37 months through 5 years of age following administration of a single booster dose of Moderna COVID-19 Vaccine included pain at the injection site, fatigue, headache, myalgia, swelling at the injection site, arthralgia, chills, axillary (or groin) swelling/tenderness, erythema at the injection site, fever, and nausea/vomiting. (See Full EUA Prescribing Information)
Adverse reactions reported in clinical trials following administration of bivalent vaccine (Original and Omicron BA.1) in individuals 18 years of age and older include pain at the injection site, fatigue, headache, myalgia, arthralgia, chills, axillary swelling/tenderness, nausea/vomiting, erythema at the injection site, swelling at the injection site, and fever. (See Full EUA Prescribing Information)
Adverse Reactions in Post-Authorization Experience of Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent
Anaphylaxis and other severe allergic reactions, myocarditis, pericarditis, syncope, and urticaria have been reported following administration of Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent during post-authorization use.
Additional adverse reactions, some of which may be serious, may become apparent with post-authorization use of Moderna COVID-19 Vaccine, Bivalent.
USE WITH OTHER VACCINES
There is no information on the co-administration of Moderna COVID-19 Vaccine, Bivalent with other vaccines.
INFORMATION TO PROVIDE TO VACCINE RECIPIENTS/CAREGIVERS
As the vaccination provider, you must communicate to the recipient or their caregiver, information consistent with the “FACT SHEET FOR RECIPIENTS AND CAREGIVERS” (and provide a copy or direct the individual to the website www.modernatx.com/covid19vaccine-eua to obtain the Fact Sheet) prior to the individual receiving each dose of Moderna COVID-19 Vaccine, Bivalent, including:
- FDA has authorized the emergency use of Moderna COVID-19 Vaccine, Bivalent, which is not an FDA-approved vaccine.
- There is an option to accept or refuse Moderna COVID-19 Vaccine, Bivalent.
- The significant known and potential risks and benefits of Moderna COVID-19 Vaccine, Bivalent, and the extent to which such risks and benefits are unknown.
- Information about available alternative vaccines and the risks and benefits of those alternatives.
For information on clinical trials that are evaluating the use of Moderna COVID-19 Vaccine, Bivalent to prevent COVID-19, please see www.clinicaltrials.gov.
Provide a vaccination card to the recipient or their caregiver.
Provide the v-safe information sheet to vaccine recipients/caregivers and encourage vaccine recipients to participate in v-safe. V-safe is a voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides dose reminders if needed and live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information, visit: www.cdc.gov/vsafe.
MANDATORY REQUIREMENTS FOR MODERNA COVID-19 VACCINE, BIVALENT ADMINISTRATION UNDER EMERGENCY USE AUTHORIZATION
In order to mitigate the risks of using this unapproved product under EUA and to optimize the potential benefit of Moderna COVID-19 Vaccine, Bivalent, the following items are required. Use of unapproved Moderna COVID-19 Vaccine, Bivalent for active immunization to prevent COVID-19 under this EUA is limited to the following (all requirements must be met):
- 1. Moderna COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months of age and older.
- 2. The vaccination provider must communicate to the individual receiving Moderna COVID-19 Vaccine, Bivalent or their caregiver information consistent with the “FACT SHEET FOR RECIPIENTS AND CAREGIVERS” prior to the individual receiving the Moderna COVID-19 Vaccine, Bivalent.
- 3. The vaccination provider must include vaccination information in the state/local jurisdiction’s Immunization Information System (IIS) or other designated system.
- 4.
The vaccination provider is responsible for mandatory reporting of the following to the Vaccine Adverse Event Reporting System (VAERS):
- vaccine administration errors whether or not associated with an adverse event,
- serious adverse events* (irrespective of attribution to vaccination),
- cases of myocarditis,
- cases of pericarditis,
- cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and
- cases of COVID-19 that result in hospitalization or death.
- Complete and submit reports to VAERS online at https://vaers.hhs.gov/reportevent.html. For further assistance with reporting to VAERS, call 1-800-822-7967. The reports should include the words “Moderna COVID-19 Vaccine, Bivalent EUA” in the description section of the report.
- 5. The vaccination provider is responsible for responding to FDA requests for information about vaccine administration errors, adverse events, cases of myocarditis, cases of pericarditis, cases of MIS in adults and children, and cases of COVID-19 that result in hospitalization or death following administration of Moderna COVID-19 Vaccine, Bivalent to recipients.
-
*Serious adverse events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
OTHER ADVERSE EVENT REPORTING TO VAERS AND MODERNATX, INC.
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to ModernaTX, Inc. using the contact information below or by providing a copy of the VAERS form to ModernaTX, Inc.
|
|
Fax number |
Telephone number |
|
1-866-599-1342 |
1-866-MODERNA (1-866-663-3762) |
ADDITIONAL INFORMATION
For general questions, visit the website or call the telephone number provided below.
To access the most recent Moderna COVID-19 Vaccine, Bivalent Fact Sheets, please scan the QR code or visit the website provided below.
|
Website |
Telephone number |
|
www.modernatx.com/covid19vaccine-eua  |
1-866-MODERNA (1-866-663-3762) |
AVAILABLE ALTERNATIVES
There may be clinical trials or availability under EUA of other COVID-19 vaccines for use as a booster dose, including bivalent vaccines that contain or encode the spike protein of the Omicron variant of SARS-CoV-2.
FEDERAL COVID-19 VACCINATION PROGRAM
This vaccine is being made available for emergency use exclusively through the CDC COVID-19 Vaccination Program (the Vaccination Program). Healthcare providers must enroll as providers in the Vaccination Program and comply with the provider requirements. Vaccination providers may not charge any fee for the vaccine and may not charge the vaccine recipient any out-of-pocket charge for administration. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, HRSA COVID-19 Uninsured Program for non-insured recipients). For information regarding provider requirements and enrollment in the CDC COVID-19 Vaccination Program, see https://www.cdc.gov/vaccines/covid-19/provider-enrollment.html.
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or TIPS.HHS.GOV.
AUTHORITY FOR ISSUANCE OF THE EUA
The Secretary of the Department of Health and Human Services (HHS) has declared a public health emergency that justifies the emergency use of drugs and biological products during the COVID-19 Pandemic. In response, the FDA has issued an EUA for the unapproved product, Moderna COVID-19 Vaccine, Bivalent, for active immunization to prevent COVID-19.
FDA issued this EUA, based on ModernaTX, Inc.’s request and submitted data.
For the authorized uses, although limited scientific information is available, based on the totality of the scientific evidence available to date, it is reasonable to believe that Moderna COVID-19 Vaccine, Bivalent may be effective for the prevention of COVID-19 in individuals as specified in the Full EUA Prescribing Information.
This EUA for Moderna COVID-19 Vaccine, Bivalent will end when the Secretary of HHS determines that the circumstances justifying the EUA no longer exist or when there is a change in the approval status of the product such that an EUA is no longer needed.
For additional information about Emergency Use Authorization, visit FDA at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy- framework/emergency-use-authorization.
COUNTERMEASURES INJURY COMPENSATION PROGRAM
The Countermeasures Injury Compensation Program (CICP) is a federal program that has been created to help pay for related costs of medical care and other specific expenses to compensate people injured after use of certain medical countermeasures. Medical countermeasures are specific vaccines, medications, devices, or other items used to prevent, diagnose, or treat the public during a public health emergency or a security threat. For more information about CICP regarding the vaccines to prevent COVID-19, visit http://www.hrsa.gov/cicp, email cicp@hrsa.gov, or call: 1-855-266-2427.
Moderna US, Inc.
Cambridge, MA 02139
©2022 ModernaTX, Inc. All rights reserved.
Patent(s): www.modernatx.com/patents
Revised: Dec/8/2022
END SHORT VERSION FACT SHEET
Long Version (Full EUA Prescribing Information) Begins On Next Page
FULL EMERGENCY USE AUTHORIZATION (EUA)
PRESCRIBING INFORMATION
MODERNA COVID-19 VACCINE, BIVALENT
(ORIGINAL AND OMICRON BA.4/BA.5)
|
FULL EUA PRESCRIBING INFORMATION: CONTENTS* 1 AUTHORIZED USE 2 DOSAGE AND ADMINISTRATION 2.1 Preparation for Administration 2.2 Administration 2.3 Dose and Schedule 3 DOSAGE FORMS AND STRENGTHS 4 CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 5.1 Management of Acute Allergic Reactions 5.2 Myocarditis and Pericarditis 5.3 Syncope 5.4 Altered Immunocompetence 5.5 Limitations of Vaccine Effectiveness 6 OVERALL SAFETY SUMMARY 6.1 Clinical Trials Experience 6.2 Post-Authorization Experience 8 REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS 10 DRUG INTERACTIONS 11 USE IN SPECIFIC POPULATIONS 11.3 Pediatric Use 11.4 Geriatric Use |
13 DESCRIPTION 14 CLINICAL PHARMACOLOGY 14.1 Mechanism of Action 18 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA 18.1 Efficacy of Two-Dose Primary Series of Moderna COVID-19 Vaccine in Participants 18 Years of Age and Older 18.2 Effectiveness of Two-Dose Primary Series in Participants 6 Months Through 5 Years of Age 18.3 Immunogenicity of the Bivalent Vaccine (Original and Omicron BA.1) Administered as a Second Booster Dose 18.4 Immunogenicity of Moderna COVID-19 Vaccine Administered as a First Booster Dose Following a Primary Series of Moderna COVID-19 Vaccine in Participants 18 Years of Age and Older 18.5 Immunogenicity of Moderna COVID-19 Vaccine Booster Dose Following Moderna COVID-19 Vaccine Primary Series in Participants 6 Months Through 5 Years of Age 19 HOW SUPPLIED/STORAGE AND HANDLING 20 PATIENT COUNSELING INFORMATION 21 CONTACT INFORMATION
|
1 AUTHORIZED USE
Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) is authorized for use under an Emergency Use Authorization (EUA) for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 6 months of age and older.
This EUA Prescribing Information pertains only to Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), hereafter referred to as Moderna COVID-19 Vaccine, Bivalent, supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box, which is authorized for use in individuals 6 months through 5 years of age.
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
The storage, preparation, and administration information in this EUA Prescribing Information apply to Moderna COVID-19 Vaccine, Bivalent for individuals 6 months through 5 years of age, which is supplied in a multiple-dose vial with dark pink cap and a label with a yellow box.
2.1 Preparation for Administration
- Moderna COVID-19 Vaccine, Bivalent multiple-dose vial with a dark pink cap and a label with a yellow box is supplied as a frozen suspension that does not contain a preservative and must be thawed prior to administration.
- Verify that the vial has a dark pink cap and a label with a yellow box and that the label states Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5).
- Thaw each vial before use following the instructions below.
- Thawing Instructions for Moderna COVID-19 Vaccine, Bivalent Multiple-Dose Vials with Dark Pink Caps and Labels with a Yellow Box
|
Thaw in Refrigerator |
Thaw at Room Temperature |
|
Thaw between 2°C to 8°C (36°F to 46°F) for 45 minutes. Let each vial stand at room temperature for 15 minutes before administering. |
Alternatively, thaw between 15°C to 25°C (59°F to 77°F) for 15 minutes. |
- After thawing, do not refreeze.
- Swirl vial gently after thawing and between each withdrawal. Do not shake. Do not dilute the vaccine.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Moderna COVID-19 Vaccine, Bivalent is a white to off-white suspension. It may contain white or translucent product-related particulates. Do not administer if vaccine is discolored or contains other particulate matter.
- A booster dose for individuals 6 months through 5 years of age and older is 0.2 mL.
- Each multiple-dose vial contains 2 booster doses of 0.2 mL.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.2 mL, discard the vial and contents. Do not pool excess vaccine from multiple vials.
- After the first booster dose has been withdrawn, the vial should be held between 2°C to 25°C (36°F to 77°F). Record the date and time of first use on the Moderna COVID-19 Vaccine, Bivalent vial label. Discard vial after 8 hours. Do not refreeze.
2.3 Dose and Schedule
Moderna COVID-19 Vaccine, Bivalent supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box is administered as a single booster dose at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine.
For individuals 6 months through 5 years of age, a single booster dose is 0.2 mL.
3 DOSAGE FORMS AND STRENGTHS
Moderna COVID-19 Vaccine, Bivalent is a suspension for injection supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box.
Each booster dose for individuals 6 months through 5 years of age is 0.2 mL.
4 CONTRAINDICATIONS
Do not administer Moderna COVID-19 Vaccine, Bivalent to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of Moderna COVID-19 Vaccine, Bivalent [see Description (13)].
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of Moderna COVID-19 Vaccine, Bivalent.
Monitor Moderna COVID-19 Vaccine, Bivalent recipients for the occurrence of immediate adverse reactions according to the Centers for Disease Control and Prevention (CDC) guidelines (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html).
5.2 Myocarditis and Pericarditis
Postmarketing safety data with Moderna COVID-19 Vaccine are relevant to Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
Postmarketing data with authorized or approved monovalent mRNA COVID-19 vaccines demonstrate increased risks of myocarditis and pericarditis, particularly within the first week following receipt of the second primary series dose or first booster dose, with most booster doses likely administered at least 5 months after completing primary vaccination. For the Moderna COVID-19 Vaccine, the observed risk is highest in males 18 through 24 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae.
The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
5.3 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines. Procedures should be in place to avoid injury from fainting.
6 OVERALL SAFETY SUMMARY
It is MANDATORY for vaccination providers to report to the Vaccine Adverse Event Reporting System (VAERS) all vaccine administration errors, all serious adverse events, cases of myocarditis, cases of pericarditis, cases of Multisystem Inflammatory Syndrome (MIS) in adults and children, and hospitalized or fatal cases of COVID-19 following vaccination with Moderna COVID-19 Vaccine, Bivalent. To the extent feasible, provide a copy of the VAERS form to ModernaTX, Inc. Please see the REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS section for details on reporting to VAERS and ModernaTX, Inc.
The safety of a booster dose of Moderna COVID-19 Vaccine, Bivalent in individuals 6 months through 5 years of age at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine is based on:
- safety data from a clinical study which evaluated a booster dose of Moderna’s bivalent COVID-19 vaccine (Original and Omicron BA.1), not authorized or approved in the U.S., hereafter referred to as bivalent vaccine (Original and Omicron BA.1),
- safety data from clinical trials which evaluated primary and booster vaccination with Moderna COVID-19 Vaccine,3 and
- postmarketing safety data with Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent.
The safety data accrued with the bivalent vaccine (Original and Omicron BA.1) and with Moderna COVID-19 Vaccine are relevant to Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.
In a clinical study, the adverse reactions in individuals 17 months through 36 months of age following administration of a single booster dose of Moderna COVID-19 Vaccine included irritability/crying (52.5%), pain at the injection site (41.7%), sleepiness (26.7%), loss of appetite (23.3%), erythema at the injection site (10.8%), swelling at the injection site (10.8%), fever (10.1%), axillary (or groin) swelling/tenderness (4.2%).
In a clinical study, the adverse reactions in individuals 37 months through 5 years of age following administration of a single booster dose of Moderna COVID-19 Vaccine included pain at the injection site (56.0%), fatigue (32.0%), headache (20.0%), myalgia (12.0%), swelling at the injection site (12.0%), arthralgia (8.0%), chills (8.0%), axillary (or groin) swelling/tenderness (4.0%), erythema at the injection site (4.0%), fever (4.0%), and nausea/vomiting (4.0%).
In a clinical study, the adverse reactions in participants 18 years of age and older following administration of a booster dose of bivalent vaccine (Original and Omicron BA.1) included pain at the injection site (77.3%), fatigue (54.9%), headache (43.9%), myalgia (39.6%), arthralgia (31.1%), chills (23.8%), axillary swelling/tenderness (17.4%), nausea/vomiting (10.3%), erythema at the injection site (6.9%), swelling at the injection site (6.9%), and fever (4.4%).
Anaphylaxis and other severe allergic reactions, myocarditis, pericarditis, syncope, and urticaria have been reported following administration of Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent outside of clinical trials.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
In five clinical trials (NCT04283461, NCT04405076, NCT04470427, NCT04649151, NCT04796896), approximately 40,000 participants aged 6 months and older received at least one dose of Moderna COVID-19 Vaccine. In one clinical trial (NCT04927065), approximately 400 participants 18 years of age and older received one dose of bivalent vaccine (Original and Omicron BA.1).
Study 1 (NCT04470427) is a Phase 3 randomized, placebo-controlled, observer-blind clinical trial conducted in the United States involving 30,346 participants 18 years of age and older who received at least one dose of Moderna COVID-19 Vaccine4 (n=15,184) or placebo (n=15,162). Study 2 (NCT04405076) is a Phase 2, randomized, observer-blind, placebo-controlled, dose-confirmation study, which included an open-label phase involving 171 participants 18 years of age and older who received a booster dose of Moderna COVID-19 Vaccine 6 months (range of 5.8 to 8.5 months) after receiving the second dose of the primary series. Study 3 (NCT04649151) is a Phase 2/3 clinical trial with multiple parts. The first portion of the trial was a randomized, placebo-controlled, observer-blind trial conducted in the United States involving 3,726 participants 12 years through 17 years of age who received at least one dose of Moderna COVID-19 Vaccine (n=2,486) or placebo (n=1,240). The trial transitioned to an open-label study in which 1,364 participants 12 years through 17 years of age received a booster dose of Moderna COVID-19 Vaccine at least 5 months after the second dose of the primary series. Study 4 (NCT04796896) is a Phase 2/3 clinical trial with multiple parts. The study includes a randomized, placebo-controlled, observer-blind clinical trial component conducted in the United States and Canada involving 10,390 participants 6 months through 11 years of age who received at least one dose of Moderna COVID-19 Vaccine (n=7,799) or placebo (n=2,591). The trial protocol was amended to include an open-label booster dose phase which included 145 participants 17 months through 5 years of age and 1,294 participants 6 years through 11 years of age who received a booster dose of Moderna COVID-19 Vaccine (10 mcg messenger RNA [mRNA] and 25 mcg mRNA, respectively) at least 6 months after completion of the Moderna COVID-19 Vaccine two-dose primary series. Study 5 (NCT04927065) is Phase 2/3 open-label study in which 437 participants 18 years of age and older, who had received a two-dose primary series and one booster dose of Moderna COVID-19 Vaccine, received a second booster dose with the bivalent vaccine (Original and Omicron BA.1) at least 3 months after the first booster dose.
Bivalent Vaccine (Original and Omicron BA.1) Administered as a Second Booster Dose
Study 5 (NCT04927065), a Phase 2/3 open-label study conducted in the United States, evaluated the immunogenicity, safety, and reactogenicity of a booster dose of the bivalent vaccine (Original and Omicron BA.1) compared to a booster dose of Moderna COVID-19 Vaccine (50 mcg mRNA; previously but no longer authorized for booster vaccination in individuals 18 years of age and older) when administered as a second booster dose to participants 18 years of age and older who had previously received a primary series and a first booster dose with Moderna COVID-19 Vaccine at least 3 months prior. The bivalent vaccine (Original and Omicron BA.1) contained 25 mcg of mRNA encoding the pre-fusion stabilized S-glycoprotein of SARS-CoV-2 Wuhan-Hu-1 strain (Original) and 25 mcg of mRNA encoding the S-glycoprotein of SARS-CoV-2 Omicron variant lineage BA.1, for a total of 50 mcg mRNA per dose. The safety analysis set included 437 participants in the bivalent vaccine (Original and Omicron BA.1) booster dose group and 377 participants in the Moderna COVID-19 Vaccine booster dose group.
The median age of the population was 60 years (range 20-96); 490 (60.2%) participants were 18 through 64 years of age and 324 (39.8%) were 65 years and older. Overall, 44.8% were male, 55.2% were female, 10.2% were Hispanic or Latino, 86.4% were White, 7.4% were African American, 3.7% were Asian, 0.1% were American Indian or Alaska Native, 0.1% were Native Hawaiian or Pacific Islander, 0.6% were other races, and 1.1% were Multiracial. Demographic characteristics were similar among participants who received the bivalent vaccine (Original and Omicron BA.1) and those who received Moderna COVID-19 Vaccine. Following the booster dose through the cutoff date of April 27, 2022, the median follow-up time was 43 days among bivalent vaccine (Original and Omicron BA.1) recipients and 57 days among Moderna COVID-19 Vaccine recipients.
Solicited Adverse Reactions
Local and systemic adverse reactions and use of antipyretic medication were solicited in an electronic diary for 7 days following each injection (i.e., day of vaccination and the next 6 days) among participants receiving bivalent vaccine (Original and Omicron BA.1) and participants receiving Moderna COVID-19 Vaccine. Events that persisted for more than 7 days were followed until resolution.
Table 1 and Table 2 present the frequency and severity of reported solicited local and systemic adverse reactions within 7 days following a second booster dose with bivalent vaccine (Original and Omicron BA.1) booster dose compared to Moderna COVID-19 Vaccine in participants 18 to <65 years of age and ≥65 years of age.
| Bivalent Vaccine
(Original and Omicron BA.1) Booster Dose (N=263) n (%) | Moderna COVID-19 Vaccine
Booster Dose (N=211) n (%) |
|
|---|---|---|
| * 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary). Solicited Safety Set consisted of participants who received a booster dose and contributed solicited adverse reaction data. Absence of rows for Grade 3 or Grade 4 adverse reactions indicates no events were reported. a Grade 3 pain and axillary swelling/tenderness: Defined as any use of prescription pain reliever; prevents daily activity. b Grade 3 swelling and erythema: Defined as >100 mm / >10 cm. c Grade 3 fatigue, myalgia, arthralgia: Defined as significant; prevents daily activity. d Grade 3 headache: Defined as significant; any use of prescription pain reliever or prevents daily activity. e Grade 3 chills: Defined as prevents daily activity and requires medical intervention. f Grade 3 fever: Defined as ≥39.0° – ≤40.0°C / ≥102.1° – ≤104.0°F. |
||
|
Local Adverse Reactions | ||
|
Pain |
231 (87.8) |
175 (82.9) |
|
Pain, Grade 3a |
2 (0.8) |
4 (1.9) |
|
Axillary swelling/tenderness |
56 (21.3) |
39 (18.5) |
|
Axillary swelling/tenderness, Grade 3a |
0 (0) |
4 (1.9) |
|
Swelling (hardness) ≥25 mm |
22 (8.4) |
15 (7.1) |
|
Swelling (hardness), Grade 3b |
4 (1.5) |
2 (0.9) |
|
Erythema (redness) ≥25 mm |
20 (7.6) |
10 (4.7) |
|
Erythema (redness), Grade 3b |
7 (2.7) |
1 (0.5) |
|
Systemic Adverse Reactions | ||
|
Fatigue |
154 (58.6) |
115 (54.5) |
|
Fatigue, Grade 3c |
10 (3.8) |
7 (3.3) |
|
Headache |
129 (49.0) |
100 (47.4) |
|
Headache, Grade 3d |
4 (1.5) |
1 (0.5) |
|
Myalgia |
113 (43.0) |
90 (42.7) |
|
Myalgia, Grade 3c |
9 (3.4) |
8 (3.8) |
|
Arthralgia |
87 (33.1) |
69 (32.7) |
|
Arthralgia, Grade 3c |
3 (1.1) |
2 (0.9) |
|
Chills |
64 (24.3) |
54 (25.6) |
|
Chills, Grade 3e |
1 (0.4) |
0 (0) |
|
Nausea/vomiting |
35 (13.3) |
27 (12.8) |
|
Fever |
10 (3.8) |
10 (4.7) |
|
Fever, Grade 3f |
1 (0.4) |
0 (0) |
|
Use of antipyretic or pain medication |
104 (39.5) |
67 (31.8) |
| Bivalent Vaccine
(Original and Omicron BA.1) Booster Dose (N=174) n (%) | Moderna COVID-19 Vaccine
Booster Dose (N=140) n (%) |
|
|---|---|---|
| * 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary). Solicited Safety Set consisted of participants who received a booster dose and contributed solicited adverse reaction data. Absence of rows for Grade 3 or Grade 4 adverse reactions indicates no events were reported. a Grade 3 pain and axillary swelling/tenderness: Defined as any use of prescription pain reliever; prevents daily activity. b Grade 3 swelling and erythema: Defined as >100 mm / >10 cm. c Grade 3 fatigue, myalgia, arthralgia: Defined as significant; prevents daily activity. d Grade 3 headache: Defined as significant; any use of prescription pain reliever or prevents daily activity. e Grade 3 chills: Defined as prevents daily activity and requires medical intervention. f Grade 3 nausea/vomiting: Defined as prevents daily activity; requires outpatient intravenous hydration. |
||
|
Local Adverse Reactions | ||
|
Pain |
107 (61.5) |
94 (67.1) |
|
Pain, Grade 3a |
2 (1.1) |
0 (0) |
|
Axillary swelling/tenderness |
20 (11.5) |
15 (10.7) |
|
Axillary swelling/tenderness, Grade 3a |
1 (0.6) |
0 (0) |
|
Swelling (hardness) ≥25 mm |
8 (4.6) |
8 (5.7) |
|
Swelling (hardness), Grade 3b |
1 (0.6) |
3 (2.1) |
|
Erythema (redness) ≥25 mm |
10 (5.7) |
3 (2.1) |
|
Erythema (redness), Grade 3b |
2 (1.1) |
1 (0.7) |
|
Systemic Adverse Reactions | ||
|
Fatigue |
86 (49.4) |
65 (46.8) |
|
Fatigue, Grade 3c |
5 (2.9) |
4 (2.9) |
|
Myalgia |
60 (34.5) |
45 (32.4) |
|
Myalgia, Grade 3c |
1 (0.6) |
5 (3.6) |
|
Headache |
63 (36.2) |
44 (31.7) |
|
Headache, Grade 3d |
1 (0.6) |
1 (0.7) |
|
Arthralgia |
49 (28.2) |
42 (30.2) |
|
Arthralgia, Grade 3c |
1 (0.6) |
1 (0.7) |
|
Chills |
40 (23.0) |
20 (14.4) |
|
Chills, Grade 3e |
0 (0) |
1 (0.7) |
|
Nausea/vomiting |
10 (5.7) |
8 (5.8) |
|
Nausea/vomiting, Grade 3f |
1 (0.6) |
0 (0) |
|
Fever |
9 (5.2) |
2 (1.4) |
|
Use of antipyretic or pain medication |
46 (26.4) |
40 (28.6) |
The median duration of solicited local and systemic adverse reactions was 2 days in participants who received either vaccine booster dose.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following the booster dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration. As of April 27, 2022, among participants who had received a booster dose (bivalent vaccine [Original and Omicron BA.1]=437, Moderna COVID-19 Vaccine=377), unsolicited adverse events that occurred within 28 days following vaccination were reported by 18.5% of participants (n=81) who received bivalent vaccine (Original and Omicron BA.1) and 20.7% of participants (n=78) who received Moderna COVID-19 Vaccine. In these analyses, 99.9% of study participants had at least 28 days of follow-up after the booster dose. The incidence of unsolicited adverse events was similar between the vaccine groups and no new safety concerns were identified.
Serious Adverse Events
As of April 27, 2022, the median duration of follow-up was 43 days among bivalent vaccine (Original and Omicron BA.1) recipients and 57 days among Moderna COVID-19 Vaccine recipients. Serious adverse events were reported by 0.7% (n=3) of participants who received bivalent vaccine (Original and Omicron BA.1) and 0.3% (n=1) of participants who received Moderna COVID-19 Vaccine. None of the events in the bivalent vaccine (Original and Omicron BA.1) group or Moderna COVID-19 Vaccine group were considered related to vaccine.
Moderna COVID-19 Vaccine Administered as a Two-Dose Primary Series
Participants 18 Years of Age and Older
The safety of Moderna COVID-19 Vaccine was evaluated in an ongoing Phase 3 randomized, placebo-controlled, observer-blind clinical trial conducted in the United States involving 30,346 participants 18 years of age and older who received at least one dose of Moderna COVID-19 Vaccine (100 mcg mRNA; n=15,184) or placebo (n=15,162) (Study 1, NCT04470427). Upon issuance of the Emergency Use Authorization (December 18, 2020) for Moderna COVID-19 Vaccine, participants were unblinded in a phased manner over a period of months to offer placebo participants Moderna COVID-19 Vaccine. The median duration of follow up for safety after the second injection during the blinded phase was 4 months. The median duration of follow up for safety after the second injection including both the blinded phase and the open-label phase was 6 months.
In Study 1, the median age of the population was 52 years (range 18-95); 22,826 (75.2%) participants were 18 to 64 years of age and 7,520 (24.8%) participants were 65 years of age and older. Overall, 52.6% of the participants were male, 47.4% were female, 20.5% were Hispanic or Latino, 79.2% were White, 10.2% were African American, 4.6% were Asian, 0.8% were American Indian or Alaska Native, 0.2% were Native Hawaiian or Pacific Islander, 2.0% were other races, and 2.1% were Multiracial. Demographic characteristics were similar between participants who received Moderna COVID-19 Vaccine and those who received placebo.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for 28 days following each dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration (2 years). Among the 30,346 participants who had received at least 1 dose of vaccine (N=15,184) or placebo (N=15,162), unsolicited adverse events that occurred within 28 days following any vaccination were reported by 31.3% of participants (n=4,752) who received Moderna COVID-19 Vaccine and 28.6% of participants (n=4,338) who received placebo.
During the 28-day follow-up period following any dose, lymphadenopathy-related events were reported by 1.7% of vaccine recipients and 0.8% of placebo recipients. These events included lymphadenopathy, lymphadenitis, lymph node pain, vaccination-site lymphadenopathy, injection-site lymphadenopathy, and axillary mass.
During the 7-day follow-up period of any vaccination, hypersensitivity events of injection site rash or injection site urticaria, likely related to vaccination, were reported by 6 participants in the Moderna COVID-19 Vaccine group and none in the placebo group. Delayed injection site reactions that began >7 days after vaccination were reported in 1.4% of vaccine recipients and 0.7% of placebo recipients. Delayed injection site reactions included pain, erythema, and swelling and are likely related to vaccination.
In the blinded portion of the study, there were 8 reports of facial paralysis (including Bell’s palsy) in the Moderna COVID-19 Vaccine group, and 3 in the placebo group. In the 28-day follow-up period there were two cases of facial paralysis in the Moderna COVID-19 Vaccine group, which occurred on 8 and 22 days, respectively, after vaccination, and one in the placebo group, which occurred 17 days after vaccination. Currently available information on facial paralysis is insufficient to determine a causal relationship with the vaccine.
In the blinded portion of the study, there were 50 reports of herpes zoster in the Moderna COVID-19 Vaccine group, and 23 in the placebo group. In the 28-day period after any vaccination, there were 22 cases of herpes zoster in the Moderna COVID-19 Vaccine group, and 15 in the placebo group. Currently available information on herpes zoster infection is insufficient to determine a causal relationship with the vaccine.
There were no other notable patterns or numerical imbalances between treatment groups for specific categories of adverse events (including other neurologic, neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Serious Adverse Events
During the blinded phase of the study, serious adverse events were reported by 1.8% (n=268) of participants who received Moderna COVID-19 Vaccine and 1.9% (n=292) of participants who received placebo.
There were three serious adverse events of angioedema/facial swelling in the vaccine group in recipients with a history of injection of dermatological fillers. The onset of swelling was reported 1-2 days after the second dose and was likely related to vaccination.
There were no other notable patterns or imbalances between treatment groups for specific categories of serious adverse events (including neurologic, neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Participants 12 Years Through 17 Years of Age
Safety data for Moderna COVID-19 Vaccine in adolescents were collected in an ongoing Phase 2/3 clinical trial with multiple parts. The first portion of the trial was a randomized, placebo-controlled, observer-blind, clinical trial conducted in the United States involving 3,726 participants 12 years through 17 years of age who received at least one dose of Moderna COVID-19 Vaccine (100 mcg mRNA; n=2,486) or placebo (n=1,240) (Study 3, NCT04649151). Overall, 51.4% were male, 48.6% were female, 11.6% were Hispanic or Latino, 83.9% were White, 3.4% were African American, 5.9% were Asian, 0.5% were American Indian or Alaska Native, <0.1% were Native Hawaiian or Pacific Islander, 1.0% were other races, and 4.5% were Multiracial. Demographic characteristics were similar among participants who received Moderna COVID-19 Vaccine and those who received placebo.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following each dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration. As of May 8, 2021, among participants who had received at least 1 dose of vaccine or placebo (vaccine=2,486, placebo=1,240), unsolicited adverse events that occurred within 28 days following each vaccination were reported by 20.5% of participants (n=510) who received Moderna COVID-19 Vaccine and 15.9% of participants (n=197) who received placebo. In these analyses, 97.3% of study participants had at least 28 days of follow-up after Dose 2.
A 14-year-old male experienced probable myocarditis with onset of symptoms 1 day after Dose 2 of Moderna COVID-19 Vaccine. Symptoms resolved after 8 days and no sequelae were observed at 5 months. There were no cases of myocarditis among placebo recipients.
During the 28-day follow-up period following any dose, lymphadenopathy-related events that were not necessarily captured in the 7-day e-diary were reported by 5.0% of vaccine recipients and 0.5% of placebo recipients. These events included lymphadenopathy, vaccination-site lymphadenopathy and injection-site lymphadenopathy which were plausibly related to vaccination.
During the 28-day follow-up period following any dose, hypersensitivity adverse events were reported in 1.8% of vaccine recipients and 0.6% of placebo recipients. Hypersensitivity events in the vaccine group included injection site rash and injection site urticaria, which are likely related to vaccination. Delayed injection site reactions that began >7 days after vaccination were reported in 0.9% of vaccine recipients and in no placebo recipients. Delayed injection site reactions included pain, erythema, and swelling and are likely related to vaccination.
There were no other notable patterns or numerical imbalances between treatment groups for specific categories of adverse events that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Serious Adverse Events
As of May 8, 2021, serious adverse events were reported by 0.2% (n=6) of participants who received Moderna COVID-19 Vaccine and 0.2% (n=2) of participants who received placebo. In these analyses, 97.3% of study participants had at least 28 days of follow-up after Dose 2, and the median follow-up time for all participants was 53 days after Dose 2.
There were no notable patterns or imbalances between treatment groups for specific categories of serious adverse events that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Additional Safety Analyses
Study 3 participants started to enter an open-label, observational phase after May 10, 2021. A long-term safety analysis was conducted in participants from Study 3 who received Moderna COVID-19 Vaccine (n=2,486) with a cut-off date of January 31, 2022. In these analyses, the median duration of follow-up including both the blinded and open-label phases was 312 days after Dose 2 and 95.6% of study participants have had at least 6 months of follow-up after Dose 2. Through the cut-off date, there were no serious adverse events causally related to the vaccine.
Participants 6 Years Through 11 Years of Age
Safety data for Moderna COVID-19 Vaccine from the blinded portion of Study 4 included data in 4,002 participants 6 years through 11 years of age who received at least one dose of Moderna COVID-19 Vaccine (50 mcg mRNA; n=3,007) or placebo (n=995). As of the data cutoff date of November 10, 2021, the median duration of blinded follow-up for safety was 51 days after Dose 2, and 1,284 participants had been followed for at least 2 months after Dose 2 (vaccine=1,006, placebo=218).
Demographic characteristics in Study 4 were similar among participants who received Moderna COVID-19 Vaccine and those who received placebo. Overall, 50.8% were male, 49.2% were female, 18.5% were Hispanic or Latino, 65.6% were White, 10.0% were African American, 9.9% were Asian, 0.4% were American Indian or Alaska Native, <0.1% were Native Hawaiian or Pacific Islander, 2.1% were other races, and 10.6% were Multiracial.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following each dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration. As of November 10, 2021, among participants who had received at least 1 dose of vaccine or placebo (vaccine=3,007, placebo=995), unsolicited adverse events that occurred within 28 days following each vaccination were reported by 29.6% of participants (n=891) who received Moderna COVID-19 Vaccine and 25.1% of participants (n=250) who received placebo. In these analyses, 98.6% of study participants had at least 28 days of follow-up after Dose 2.
During the 28-day follow-up period following any dose, lymphadenopathy-related events were reported by 1.8% of vaccine recipients and 0.6% of placebo recipients. These events included lymphadenopathy, lymph node pain, injection-site lymphadenopathy, and vaccination-site lymphadenopathy which were plausibly related to vaccination.
During the 28-day follow-up period following any dose, hypersensitivity adverse events were reported in 4.3% of vaccine recipients and 2.1% of placebo recipients. Hypersensitivity events in the vaccine group included injection site rash and injection site urticaria, which are likely related to vaccination. Delayed injection site reactions that began >7 days after vaccination were reported in 2.7% of vaccine recipients and in 0.2% of placebo recipients. Delayed injection site reactions included pain, erythema, and swelling and are likely related to vaccination.
During the 28-day follow-up period following any dose, events of abdominal pain (including abdominal pain, abdominal pain upper, and abdominal pain lower) were reported by 1.1% of vaccine recipients and 0.6% of placebo recipients. Currently available information is insufficient to determine a causal relationship with the vaccine.
There were no other notable patterns or numerical imbalances between treatment groups for specific categories of adverse events that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Serious Adverse Events
As of November 10, 2021, serious adverse events were reported by 0.2% (n=6) of participants who received Moderna COVID-19 Vaccine and 0.2% (n=2) participants who received placebo. None of the events in the Moderna COVID-19 Vaccine group were considered related to vaccine. In these analyses, 98.6% of study participants had at least 28 days of follow-up after Dose 2, and the median follow-up time for all participants was 51 days after Dose 2.
There were no notable patterns or imbalances between treatment groups for specific categories of serious adverse events that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Additional Safety Analyses
Participants 6 years through 11 years in Study 4 started to enter an open-label, observational phase after November 1, 2021. A long-term safety analysis was conducted in participants 6 years through 11 years from Study 4 who received Moderna COVID-19 Vaccine (n=3,007) with a cut-off date of February 21, 2022. In these analyses, the median duration of follow-up including both the blinded and open-label phases was 158 days after Dose 2. Through the cut-off date, there were no serious adverse events causally related to the vaccine.
Participants 6 Months Through 5 Years of Age
Safety data for Moderna COVID-19 Vaccine from the blinded portion of Study 4 included data in 6,388 participants 6 months through 5 years of age who received at least one dose of Moderna COVID-19 Vaccine (25 mcg mRNA; n=4,792) or placebo (n=1,596). As of the data cutoff date of February 21, 2022, the median duration of blinded follow-up for safety for participants 6 months through 23 months was 68 days after Dose 2. For participants 2 years to 5 years, the median duration of blinded follow-up for safety was 71 days after Dose 2.
For participants 6 months through 23 months, 51.1% were male, 48.9% were female, 13.2% were Hispanic or Latino, 79.0% were White, 3.1% were African American, 4.9% were Asian, 0.2% were American Indian or Alaska Native, 0.0% were Native Hawaiian or Pacific Islander, 1.5% were other races, and 10.6% were Multiracial. For participants 2 years through 5 years, 50.8% were male, 49.2% were female, 14.2% were Hispanic or Latino, 76.5% were White, 4.5% were African American, 6.0% were Asian, 0.4% were American Indian or Alaska Native, 0.3% were Native Hawaiian or Pacific Islander, 1.5% were other races, and 10.4% were Multiracial. Demographic characteristics were similar among participants who received Moderna COVID-19 Vaccine and those who received placebo.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following each dose and follow-up is ongoing. Serious adverse events and medically attended adverse events will be recorded for the entire study duration.
As of February 21, 2022, among participants 6 months through 23 months of age who had received at least 1 dose of vaccine or placebo (vaccine=1,761, placebo=589), unsolicited adverse events that occurred within 28 days following each vaccination were reported by 49.3% of participants (n=869) who received Moderna COVID-19 Vaccine and 48.2% of participants (n=284) who received placebo. In these analyses, 83.1% of study participants 6 months through 23 months of age had at least 28 days of follow-up after Dose 2. Among participants 2 years through 5 years of age who had received at least 1 dose of vaccine or placebo (vaccine=3,031, placebo=1,007), unsolicited adverse events that occurred within 28 days following each vaccination were reported by 40.0% of participants (n=1,212) who received Moderna COVID-19 Vaccine and 37.5% of participants (n=378) who received placebo. In these analyses, 89.3% of study participants 2 years through 5 years of age had at least 28 days of follow-up after Dose 2.
During the 28-day follow-up period following any dose, lymphadenopathy-related events were reported by 1.5% of vaccine recipients and 0.2% of placebo recipients who were 6 months through 23 months of age and 0.9% of vaccine recipients and <0.1% of placebo recipients who were 2 years through 5 years of age. These events included lymphadenopathy, injection-site lymphadenopathy, and vaccination-site lymphadenopathy which were plausibly related to vaccination.
During the 28-day follow-up period following any dose, hypersensitivity adverse events were reported in 3.9% of vaccine recipients and 5.3% of placebo recipients who were 6 months through 23 months of age and 3.5% of vaccine recipients and 2.5% of placebo recipients who were 2 years through 5 years of age. Hypersensitivity events in the vaccine group included injection site rash and injection site urticaria, which are likely related to vaccination. Delayed injection site reactions that began >7 days after vaccination were reported in 1.2% of vaccine recipients and no placebo recipients who were 6 months through 23 months of age and 1.4% of vaccine recipients and <0.1% of placebo recipients who were 2 years through 5 years of age. Delayed injection site reactions included pain, erythema, and swelling and are likely related to vaccination.
During the 28-day follow-up period following any dose, events of abdominal pain (including abdominal pain, abdominal pain upper, and abdominal discomfort) were reported by 0.7% of vaccine recipients and 0.4% of placebo recipients who were 2 years through 5 years of age. Currently available information is insufficient to determine a causal relationship with the vaccine.
There were no other notable patterns or numerical imbalances between treatment groups for specific categories of adverse events that would suggest a causal relationship to Moderna COVID-19 Vaccine.
Serious Adverse Events
As of February 21, 2022, serious adverse events were reported by 0.9% (n=15) of participants who received vaccine and 0.2% (n=1) of participants who received placebo who were 6 months through 23 months of age and 0.3% (n=9) of participants who received Moderna COVID-19 Vaccine and 0.2% (n=2) of participants who received placebo who were 2 years through 5 years of age. In these analyses, 83.1% of study participants 6 months through 23 months of age had at least 28 days of follow-up after Dose 2, and the median follow-up time for all participants was 68 days after Dose 2. In these analyses, 89.3% of study participants 2 years through 5 years of age had at least 28 days of follow-up after Dose 2, and the median follow-up time for all participants was 71 days after Dose 2.
In participants 6 months through 23 months of age who received the vaccine, a 1-year-old female experienced serious adverse events of a Grade 3 fever 6 hours after Dose 1 and a febrile convulsion 1 day after Dose 1. These events were considered related to vaccination. In participants 2 years through 5 years of age who received Moderna COVID-19 Vaccine, none of the events were considered related to vaccine.
Moderna COVID-19 Vaccine Administered as a First Booster Dose Following a Primary Series of Moderna COVID-19 Vaccine
Participants 18 Years of Age and Older
Study 2 is a Phase 2, randomized, observer-blind, placebo-controlled, dose-confirmation study to evaluate the safety, reactogenicity, and immunogenicity of Moderna COVID-19 Vaccine in participants 18 years of age and older (NCT04405076). In this study, 198 participants received two doses (0.5 mL 1 month apart) of Moderna COVID-19 Vaccine primary series. In an open label-phase, 171 of those participants received a single booster dose (50 mcg mRNA; 0.25 mL) at least 6 months (range of 5.8 to 8.5 months) after receiving the second dose of the primary series.
Among the 171 booster dose recipients, the median age was 55 years (range 18-87), 39.2% were male and 60.8% were female, 95.9% were White, 5.8% were Hispanic or Latino, 2.9% were Black or African American, 0.6% were Asian, and 0.6% were American Indian or Alaska Native. Following the booster dose, the median follow-up time was 5.7 months (range of 3.1 to 6.4 months).
Unsolicited Adverse Events
Overall, the 171 participants who received a booster dose had a median follow-up time of 5.7 months after the booster dose to the cut-off date (August 16, 2021). Through the cut-off date, there were no unsolicited adverse events not already captured as solicited local and systemic reactions that were considered causally related to Moderna COVID-19 Vaccine.
Serious Adverse Events
Of the 171 participants who received a booster dose of Moderna COVID-19 Vaccine, there were no serious adverse events reported from the booster dose through 28 days after the booster dose. Through the cut-off date of August 16, 2021, there were no serious adverse events following the booster dose considered causally related to Moderna COVID-19 Vaccine.
Participants 12 Years Through 17 Years of Age
Safety data for a booster dose of Moderna COVID-19 Vaccine in adolescents were collected in an ongoing Phase 2/3 clinical trial with multiple parts. The open-label booster portion of the study involved 1,364 participants 12 years through 17 years of age who received a booster dose of Moderna COVID-19 Vaccine (50 mcg mRNA; 0.25 mL) at least 5 months after the second dose of the primary series (Study 3, NCT04649151). Overall, 51.2% were male, 48.8% were female, 13.1% were Hispanic or Latino, 84.9% were White, 3.2% were African American, 4.8% were Asian, 0.5% were American Indian or Alaska Native, <0.1% were Native Hawaiian or Pacific Islander, 0.7% were other races, and 5.2% were Multiracial. As of the data cutoff date of May 16, 2022, the median duration of follow-up for safety was 116 days after the booster dose.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following the booster dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration. As of May 16, 2022, among the 1,364 participants who had received a booster dose, unsolicited adverse events that occurred within 28 days following vaccination were reported by 14.2% of participants (n=194). In these analyses, 97.4% of study participants had at least 28 days of follow-up after the booster dose. No new safety concerns were identified.
Serious Adverse Events
Through the cut-off date of May 16, 2022, with a median follow-up duration of 116 days after booster, no serious adverse events following the booster dose were reported.
Participants 6 Years Through 11 Years of Age
Safety data for a booster dose of Moderna COVID-19 Vaccine in individuals 6 years through 11 years of age were collected in an ongoing Phase 2/3 clinical trial with multiple parts. The open-label booster portion of the study involved 1,294 participants 6 years through 11 years of age who received a booster dose of Moderna COVID-19 Vaccine (25 mcg mRNA) at least 6 months after the second dose of the primary series (Study 4, NCT04796896). Overall, 51.9%% were male, 48.1% were female, 15.6% were Hispanic or Latino, 65.7% were White, 11.0% were African American, 7.8% were Asian, 0.5% were American Indian or Alaska Native, <0.1% were Native Hawaiian or Pacific Islander, 1.9% were other races, and 11.8% were Multiracial. As of the data cutoff date of May 23, 2022, the median duration of follow-up for safety was 29 days after the booster dose.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following the booster dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration. As of May 23, 2022, among the 1,294 participants who had received a booster dose, unsolicited adverse events that occurred within 28 days following vaccination were reported by 13.1% of participants (n=169). In these analyses, 55.4% of study participants had at least 28 days of follow-up after the booster dose. Serum sickness-like reaction with onset 10 days following administration of a booster dose was reported in an 8-year-old participant. This event was assessed as related to vaccination. After initiation of treatment with antihistamines and steroids, symptoms resolved within 15 days with the exception of intermittent urticaria that was ongoing 31 days after the onset of the reaction.
Serious Adverse Events
As of May 23, 2022, with a median follow-up duration of 29 days after booster, there was one serious adverse event of abdominal pain reported 16 days following booster dose by a 7-year-old participant. Currently available information is insufficient to determine a causal relationship with the vaccine.
Participants 17 Months Through 5 Years of Age
Safety data in support of a booster dose of Moderna COVID-19 Vaccine in individuals 6 months through 5 years of age were collected in participants 17 months through 5 years of age at the time of the booster dose in an ongoing Phase 2/3 clinical trial with multiple parts. The open-label booster portion of the study involved 145 participants 17 months through 5 years of age who received a booster dose of Moderna COVID-19 Vaccine (10 mcg mRNA) at least 6 months (range 8-13 months; median 10 months) after the completion of the Moderna COVID-19 Vaccine two-dose primary series (Study 4, NCT04796896). Overall, 55.2% were male, 44.8% were female, 10.3% were Hispanic or Latino, 80.0% were White, 2.8% were African American, 6.2% were Asian, 0.7% were American Indian or Alaska Native, 0.0% were Native Hawaiian or Pacific Islander, 2.8% were other races, and 7.6% were Multiracial. As of the data cutoff date of August 18, 2022, the median duration of follow-up for safety after the booster dose was 99 days.
Solicited Adverse Reactions
Local and systemic adverse reactions and use of antipyretic medication were solicited in an electronic diary for 7 days following the injection (i.e., day of vaccination and the next 6 days) among participants receiving Moderna COVID-19 Vaccine (10 mcg mRNA). Events that persisted for more than 7 days were followed until resolution.
The frequency and severity of reported solicited local and systemic adverse reactions within 7 days of a booster vaccination among participants 17 months through 36 months are presented in Table 3, and among participants 37 months through 5 years are presented in Table 4.
| Moderna COVID-19 Vaccine Booster Dose
(N=120†) n (%) |
|
|---|---|
| * 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary). Absence of rows for Grade 3 or Grade 4 adverse reactions indicates no events were reported. † Four participants were older than 36 months of age at the time of the booster dose; however, solicited adverse reactions were collected and graded using the diary card and grading scale for participants 6 months through 36 months of age. |
|
|
Local Adverse Reactions | |
|
Pain |
50 (41.7) |
|
Erythema (redness) ≥5 mm |
13 (10.8) |
|
Erythema (redness) Grade 3: >50 mm |
1 (0.8) |
|
Swelling (hardness) ≥5 mm |
13 (10.8) |
|
Axillary (or groin) swelling/tenderness |
5 (4.2) |
|
Systemic Adverse Reactions | |
|
Irritability/crying |
63 (52.5) |
|
Sleepiness |
32 (26.7) |
|
Loss of appetite |
28 (23.3) |
|
Fever >38.0°C / >100.4°F |
12 (10.1) |
|
Fever, Grade 3: 39.6° - 40.0°C / 103.2° - 104.0°F |
2 (1.7) |
|
Fever, Grade 4: >40.0°C / >104.0°F |
1 (0.8) |
|
Use of antipyretic or pain medication |
24 (20.0) |
| Moderna COVID-19 Vaccine Booster Dose
(N=25) n (%) |
|
|---|---|
| * 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary). Absence of rows for Grade 3 or Grade 4 adverse reactions indicates no events were reported. | |
|
Local Adverse Reactions | |
|
Pain |
14 (56.0) |
|
Swelling (hardness) ≥25 mm |
3 (12.0) |
|
Axillary (or groin) swelling/tenderness |
1 (4.0) |
|
Erythema (redness) ≥25 mm |
1 (4.0) |
|
Systemic Adverse Reactions | |
|
Fatigue |
8 (32.0) |
|
Headache |
5 (20.0) |
|
Myalgia |
3 (12.0) |
|
Arthralgia |
2 (8.0) |
|
Chills |
2 (8.0) |
|
Fever >38.0°C / >100.4°F |
1 (4.0) |
|
Fever, Grade 3: 39.0° - 40.0°C / 102.1° - 104.0°F |
1 (4.0) |
|
Nausea/vomiting |
1 (4.0) |
|
Use of antipyretic or pain medication |
6 (24.0) |
In participants who received a booster dose, the median duration of solicited local and systemic adverse reactions was 3 days.
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for up to 28 days following the booster dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration. As of August 18, 2022, among the 145 participants who had received a booster dose, unsolicited adverse events that occurred within 28 days following vaccination were reported by 24.1% of participants (n=35). In these analyses, 99.3% of study participants had at least 28 days of follow-up. Through the cut-off date, there were no unsolicited adverse events not already captured as solicited local and systemic reactions that were considered causally related to Moderna COVID-19 Vaccine.
Serious Adverse Events
As of August 18, 2022, with a median follow-up duration after the booster dose of 99 days, there were no serious adverse events reported following the booster dose.
6.2 Post-Authorization Experience
The following adverse reactions have been identified during post-authorization use of Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent. Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Immune System Disorders: anaphylaxis, urticaria
Nervous System Disorders: syncope
8 REQUIREMENTS AND INSTRUCTIONS FOR REPORTING ADVERSE EVENTS AND VACCINE ADMINISTRATION ERRORS
See Overall Safety Summary (Section 6) for additional information.
The vaccination provider enrolled in the federal COVID-19 Vaccination Program is responsible for the MANDATORY reporting of the listed events following administration of the Moderna COVID-19 Vaccine, Bivalent to the Vaccine Adverse Event Reporting System (VAERS)
- Vaccine administration errors whether or not associated with an adverse event
- Serious adverse events* (irrespective of attribution to vaccination)
- Cases of myocarditis
- Cases of pericarditis
- Cases of Multisystem Inflammatory Syndrome (MIS) in adults and children
- Cases of COVID-19 that results in hospitalization or death
*Serious Adverse Events are defined as:
- Death;
- A life-threatening adverse event;
- Inpatient hospitalization or prolongation of existing hospitalization;
- A persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions;
- A congenital anomaly/birth defect;
- An important medical event that based on appropriate medical judgement may jeopardize the individual and may require medical or surgical intervention to prevent one of the outcomes listed above.
Instructions for Reporting to VAERS
The vaccination provider enrolled in the federal COVID-19 Vaccination Program should complete and submit a VAERS form to FDA using one of the following methods:
- Complete and submit the report online: https://vaers.hhs.gov/reportevent.html, or
- If you are unable to submit this form electronically, you may fax it to VAERS at 1-877-721-0366. If you need additional help submitting a report, you may call the VAERS toll-free information line at 1-800-822-7967 or send an email to info@vaers.org.
IMPORTANT: When reporting adverse events or vaccine administration errors to VAERS, please complete the entire form with detailed information. It is important that the information reported to FDA be as detailed and complete as possible. Information to include:
- Patient demographics (e.g., patient name, date of birth)
- Pertinent medical history
- Pertinent details regarding admission and course of illness
- Concomitant medications
- Timing of adverse event(s) in relationship to administration of Moderna COVID-19 Vaccine, Bivalent
- Pertinent laboratory and virology information
- Outcome of the event and any additional follow-up information if it is available at the time of the VAERS report. Subsequent reporting of follow-up information should be completed if additional details become available.
The following steps are highlighted to provide the necessary information for safety tracking:
- 1. In Box 17, provide information on Moderna COVID-19 Vaccine, Bivalent and any other vaccines administered on the same day; and in Box 22, provide information on any other vaccines received within one month prior.
- 2.
In Box 18, description of the event:
- a. Write “Moderna COVID-19 Vaccine, Bivalent EUA” as the first line
- b. Provide a detailed report of vaccine administration error and/or adverse event. It is important to provide detailed information regarding the patient and adverse event/medication error for ongoing safety evaluation of this unapproved vaccine. Please see information to include listed above.
- 3.
Contact information:
- a. In Box 13, provide the name and contact information of the prescribing healthcare provider or institutional designee who is responsible for the report.
- b. In Box 14, provide the name and contact information of the best doctor/healthcare professional to contact about the adverse event.
- c. In Box 15, provide the address of the facility where vaccine was given (NOT the healthcare provider’s office address).
Other Reporting Instructions
Vaccination providers may report to VAERS other adverse events that are not required to be reported using the contact information above.
To the extent feasible, report adverse events to ModernaTX, Inc. using the contact information below or by providing a copy of the VAERS form to ModernaTX, Inc.
|
|
Fax number |
Telephone number |
|
1-866-599-1342 |
1-866-MODERNA (1-866-663-3762) |
10 DRUG INTERACTIONS
There are no data to assess the concomitant administration of Moderna COVID-19 Vaccine, Bivalent with other vaccines.
11 USE IN SPECIFIC POPULATIONS
11.3 Pediatric Use
Moderna COVID-19 Vaccine, Bivalent is authorized for use in individuals 6 months through 17 years of age. This authorization is based on safety and effectiveness data with Moderna COVID-19 Vaccine in individuals 6 months of age and older, and safety and immunogenicity data with the bivalent vaccine (Original and Omicron BA.1) in adults.
Moderna COVID-19 Vaccine, Bivalent is not authorized for use in individuals younger than 6 months of age.
11.4 Geriatric Use
Clinical studies of Moderna COVID-19 Vaccine and the bivalent vaccine (Original and Omicron BA.1) included participants 65 years of age and older, and their data contribute to the overall assessment of safety and effectiveness of Moderna COVID-19 Vaccine, Bivalent. [see Overall Safety Summary (6.1) and Clinical Trial Results and Supporting Data for EUA (18.1)]. Some local and systemic adverse reactions were reported in a lower proportion of participants 65 years of age and older compared to participants 18 through 64 years of age [see Overall Safety Summary (6.1)].
In an ongoing Phase 3 clinical study (Study 1) of primary series dosing of Moderna COVID-19 Vaccine, 24.8% (n=7,520) of participants were 65 years of age and older and 4.6% (n=1,399) of participants were 75 years of age and older.
In an ongoing Phase 2/3 clinical study (Study 5) of a single booster dose of bivalent vaccine (Original and BA.1), 39.8% (n=174) were 65 years of age and older
In a Phase 2 clinical study (Study 2) of a single booster dose of Moderna COVID-19 Vaccine, 22.2% (n=38) of participants were 65 years of age and older.
13 DESCRIPTION
Moderna COVID-19 Vaccine, Bivalent is provided as a sterile white to off-white suspension for intramuscular injection.
Each 0.2 mL booster dose of Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), supplied in a multiple-dose vial with a dark pink cap and a label with a yellow box, contains 5 mcg nucleoside-modified messenger RNA (mRNA) encoding the pre-fusion stabilized Spike glycoprotein (S) of the SARS-CoV-2 Wuhan-Hu-1 strain (Original) and 5 mcg mRNA encoding the pre-fusion stabilized S-protein of the SARS-CoV-2 Omicron variant lineages BA.4 and BA.5 (Omicron BA.4/BA.5). The S-proteins of the SARS-CoV-2 Omicron variant lineages BA.4 and BA.5 are identical. Each dose also contains the following ingredients: a total lipid content of 0.20 mg (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), 0.09 mg tromethamine, 0.51 mg tromethamine hydrochloride, 0.0042 mg acetic acid, 0.02 mg sodium acetate trihydrate, and 17.4 mg sucrose.
Moderna COVID-19 Vaccine, Bivalent does not contain a preservative.
The vial stoppers are not made with natural rubber latex.
14 CLINICAL PHARMACOLOGY
14.1 Mechanism of Action
The nucleoside-modified mRNA in Moderna COVID-19 Vaccine, Bivalent is formulated in lipid particles, which enable delivery of the nucleoside-modified mRNA into host cells to allow expression of the SARS-CoV-2 S antigen. The vaccine elicits an immune response to the S antigen, which protects against COVID-19
18 CLINICAL TRIAL RESULTS AND SUPPORTING DATA FOR EUA
The effectiveness of a booster dose of Moderna COVID-19 Vaccine, Bivalent is based on effectiveness of primary and booster vaccination with Moderna COVID-19 Vaccine and immunogenicity of a second booster dose with the bivalent vaccine (Original and Omicron BA.1).
18.1 Efficacy of Two-Dose Primary Series of Moderna COVID-19 Vaccine in Participants 18 Years of Age and Older
Study 1 is an ongoing Phase 3 randomized, placebo-controlled, observer-blind clinical trial to evaluate the efficacy, safety, and immunogenicity of the Moderna COVID-19 Vaccine in participants 18 years of age and older in the United States (NCT04470427). Randomization was stratified by age and health risk: 18 to <65 years of age without comorbidities (not at risk for progression to severe COVID-19), 18 to <65 years of age with comorbidities (at risk for progression to severe COVID-19), and 65 years of age and older with or without comorbidities. Participants who were immunocompromised and those with a known history of SARS-CoV-2 infection were excluded from the study. Participants with no known history of SARS-CoV-2 infection but with positive laboratory results indicative of infection at study entry were included. The study allowed for the inclusion of participants with stable pre-existing medical conditions, defined as disease not requiring significant change in therapy or hospitalization for worsening disease during the 3 months before enrollment, as well as participants with stable human immunodeficiency virus (HIV) infection. A total of 30,420 participants were randomized equally to receive 2 doses of the Moderna COVID-19 Vaccine (100 mcg mRNA) or saline placebo 1 month apart. Participants will be followed for efficacy and safety until 24 months after the second dose.
The primary efficacy analysis population (referred to as the Per-Protocol Set) included 28,207 participants who received two doses (0.5 mL at 0 and 1 month) of either Moderna COVID-19 Vaccine (100 mcg mRNA; n=14,134) or placebo (n=14,073) and had a negative baseline SARS-CoV-2 status. In the Per-Protocol Set, 47.4% were female, 19.7% were Hispanic or Latino; 79.5% were White, 9.7% were African American, 4.6% were Asian, and 2.1% other races. The median age of participants was 53 years (range 18-95) and 25.3% of participants were 65 years of age and older. Of the study participants in the Per-Protocol Set, 18.5% were at increased risk of severe COVID-19 due to at least one pre-existing medical condition (chronic lung disease, significant cardiac disease, severe obesity, diabetes, liver disease, or HIV infection) regardless of age. Between participants who received Moderna COVID-19 Vaccine and those who received placebo, there were no notable differences in demographics or pre-existing medical conditions.
Efficacy Against COVID-19
COVID-19 was defined based on the following criteria: The participant must have experienced at least two of the following systemic symptoms: fever (≥38ºC / ≥100.4°F), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s); or the participant must have experienced at least one of the following respiratory signs/symptoms: cough, shortness of breath or difficulty breathing, or clinical or radiographical evidence of pneumonia; and the participant must have at least one NP swab, nasal swab, or saliva sample (or respiratory sample, if hospitalized) positive for SARS-CoV-2 by RT-PCR. COVID-19 cases were adjudicated by a Clinical Adjudication Committee.
The median length of follow-up for efficacy for participants in the study was 9 weeks post-Dose 2. There were 11 COVID-19 cases in the Moderna COVID-19 Vaccine group and 185 cases in the placebo group, with a vaccine efficacy of 94.1% (95% confidence interval of 89.3% to 96.8%).
| * COVID-19: symptomatic COVID-19 requiring positive RT-PCR result and at least two systemic symptoms or one respiratory symptom. Cases starting 14 days after Dose 2. † VE and 95% CI from the stratified Cox proportional hazard model. |
||||||
|
Moderna COVID-19 Vaccine |
Placebo |
% Vaccine Efficacy (95% CI)† |
||||
|
Participants (N) |
COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
Participants (N) |
COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
|
|
14,134 |
11 |
3.328 |
14,073 |
185 |
56.510 |
94.1 (89.3, 96.8) |
The subgroup analyses of vaccine efficacy are presented in Table 6.
| * COVID-19: symptomatic COVID-19 requiring positive RT-PCR result and at least two systemic symptoms or one respiratory symptom. Cases starting 14 days after Dose 2. † VE and 95% CI from the stratified Cox proportional hazard model. |
|||||||
|
Age Subgroup (Years) |
Moderna COVID-19 Vaccine |
Placebo |
% Vaccine Efficacy (95% CI)† |
||||
|
Participants (N) |
COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
Participants (N) |
COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
||
|
18 to <65 |
10,551 |
7 |
2.875 |
10,521 |
156 |
64.625 |
95.6 (90.6, 97.9) |
|
≥65 |
3,583 |
4 |
4.595 |
3,552 |
29 |
33.728 |
86.4 (61.4, 95.2) |
Severe COVID-19 was defined based on confirmed COVID-19 as per the primary efficacy endpoint case definition, plus any of the following: Clinical signs indicative of severe systemic illness, respiratory rate ≥30 per minute, heart rate ≥125 beats per minute, SpO2 ≤93% on room air at sea level or PaO2/FIO2 <300 mm Hg; or respiratory failure or ARDS (defined as needing high-flow oxygen, non-invasive or mechanical ventilation, or ECMO), evidence of shock (systolic blood pressure <90 mmHg, diastolic BP <60 mmHg or requiring vasopressors); or significant acute renal, hepatic, or neurologic dysfunction; or admission to an intensive care unit or death.
Among all participants in the Per-Protocol Set analysis, which included COVID-19 cases confirmed by an adjudication committee, no cases of severe COVID-19 were reported in the Moderna COVID-19 Vaccine group compared with 30 cases reported in the placebo group (incidence rate 9.138 per 1,000 person-years). One PCR-positive case of severe COVID-19 in a vaccine recipient was awaiting adjudication at the time of the analysis.
18.2 Effectiveness of Two-Dose Primary Series in Participants 6 Months Through 5 Years of Age
Study 4 includes an ongoing Phase 2/3 randomized, placebo-controlled, observer-blind, clinical trial component to evaluate the safety, reactogenicity, and effectiveness of the Moderna COVID-19 Vaccine in individuals ages 6 months through 5 years in the United States and Canada (NCT04796896). Participants with a known history of SARS-CoV-2 infection within 2 weeks of study vaccination were excluded from the study. A total of 6,403 participants were randomized 3:1 to receive 2 doses of the Moderna COVID-19 Vaccine (25 mcg mRNA per dose) or saline placebo 1 month apart. Participants will be followed for occurrence of COVID-19 and safety until 1 year after the last dose.
Effectiveness in individuals 6 months through 5 years of age is based on a comparison of immune responses in this age group to adults 18 years through 25 years of age.
In Study 4, an analysis was conducted of SARS-CoV-2 neutralizing antibody concentrations and seroresponse rates 28 days after Dose 2 in a subset of individuals 6 months through 5 years of age in Study 4 and participants 18 years through 25 years of age in Study 1 who had no immunologic or virologic evidence of prior SARS-CoV-2 at baseline. Noninferior immune responses as assessed by neutralizing antibody concentrations in arbitrary units (AU)/mL and seroresponse rates were demonstrated in a comparison of individuals 6 months through 23 months of age to participants 18 years through 25 years of age (Table 7) and 2 years through 5 years of age to participants 18 years through 25 years of age (Table 8).
| GMC = Geometric mean concentration n = number of participants with non-missing data at baseline and at Day 57 * Antibody values reported as below the lower limit of quantification (LLOQ) are replaced by 0.5 x LLOQ. Values greater than the upper limit of quantification (ULOQ) are replaced by the ULOQ if actual values are not available. a The log-transformed antibody levels are analyzed using an analysis of covariance (ANCOVA) model with the group variable (individuals in Study 4 and young adults in Study 1) as fixed effect. The resulted LS means, difference of LS means, and 95% CI are back transformed to the original scale for presentation. b Noninferiority is declared if the lower bound of the 2-sided 95% CI for the GMC ratio is greater than 0.67, with a point estimate of >0.8 and the lower bound of the 2-sided 95% CI for difference in seroresponse rate is greater than -10%, with a point estimate of >-5%. c Final geometric mean antibody concentrations (GMC) in AU/mL were determined using SARS-CoV-2 microneutralization assay. The SARS CoV-2 MN is a cell-based assay that is designed to determine the ability of SARS-CoV-2 neutralizing antibodies to inhibit the infection of 293T-ACE2 cells by SARS-CoV-2 Reporter Virus Particles (RVP) which express green fluorescent protein (GFP). A given serum sample is pre-incubated with a known quantity of SARS-CoV-2-GFP for 60 (±5) minutes prior to infection of 293T-ACE2 cells. COVID-19 infection is monitored 48 (±4) hours following infection by counting the number of green fluorescent cells using the Cytation 5 cell imaging reader. d Seroresponse due to vaccination specific to SARS-CoV-2 RVP neutralizing antibody concentration at a subject level is defined in protocol as a change from below LLOQ to equal or above 4 x LLOQ, or at least a 4-fold rise if baseline is equal to or above LLOQ. Seroresponse 95% CI is calculated using the Clopper-Pearson method. |
|||||
| e Difference in seroresponse rate 95% CI is calculated using the Miettinen-Nurminen (score) confidence limits. | |||||
|
Moderna COVID-19 Vaccine |
|||||
|
6 Months Through n=230 |
18 Years Through n=291 |
6 Months Through 23 Months/ 18 Years Through 25 Years |
|||
|
Assay |
Time Point |
GMC (95% CI)* |
GMC (95% CI)* |
GMC Ratio (95% CI)a |
Met Noninferiority Objective (Y/N)b |
|
SARS-CoV-2 neutralization assayc |
28 days after Dose 2 |
1780.7 (1606.4, 1973.8) |
1390.8 (1269.1, 1524.2) |
1.3 (1.1, 1.5) |
Y |
|
% (95% CI)d |
% (95% CI)d |
(95% CI)e |
|||
|
100 (98.4, 100) |
99.3 (97.5, 99.9) |
0.7 (-1.0, 2.5) |
|||
| GMC = Geometric mean concentration n = number of participants with non-missing data at baseline and at Day 57 * Antibody values reported as below the lower limit of quantification (LLOQ) are replaced by 0.5 x LLOQ. Values greater than the upper limit of quantification (ULOQ) are replaced by the ULOQ if actual values are not available. a The log-transformed antibody levels are analyzed using an analysis of covariance (ANCOVA) model with the group variable (individuals in Study 4 and young adults in Study 1) as fixed effect. The resulted LS means, difference of LS means, and 95% CI are back transformed to the original scale for presentation. b Noninferiority is declared if the lower bound of the 2-sided 95% CI for the GMC ratio is greater than 0.67, with a point estimate of >0.8 and the lower bound of the 2-sided 95% CI for difference in seroresponse rate is greater than -10%, with a point estimate of >-5%. c Final geometric mean antibody concentrations (GMC) in AU/mL were determined using SARS-CoV-2 microneutralization assay. The SARS CoV-2 MN is a cell-based assay that is designed to determine the ability of SARS-CoV-2 neutralizing antibodies to inhibit the infection of 293T-ACE2 cells by SARS-CoV-2 Reporter Virus Particles (RVP) which express green fluorescent protein (GFP). A given serum sample is pre-incubated with a known quantity of SARS-CoV-2-GFP for 60 (±5) minutes prior to infection of 293T-ACE2 cells. COVID-19 infection is monitored 48 (±4) hours following infection by counting the number of green fluorescent cells using the Cytation 5 cell imaging reader. d Seroresponse due to vaccination specific to SARS-CoV-2 RVP neutralizing antibody concentration at a subject level is defined in protocol as a change from below LLOQ to equal or above 4 x LLOQ, or at least a 4-fold rise if baseline is equal to or above LLOQ. Seroresponse 95% CI is calculated using the Clopper-Pearson method. |
|||||
| e Difference in seroresponse rate 95% CI is calculated using the Miettinen-Nurminen (score) confidence limits. | |||||
|
Moderna COVID-19 Vaccine |
|||||
|
2 Years Through n=264 |
18 Years Through n=291 |
2 Years Through 5 Years/ 18 Years Through 25 Years |
|||
|
Assay |
Time Point |
GMC (95% CI)* |
GMC (95% CI)* |
GMC Ratio (95% CI)a |
Met Noninferiority Objective (Y/N)b |
|
SARS-CoV-2 neutralization assayc |
28 days after Dose 2 |
1410.0 (1273.8, 1560.8) |
1390.8 (1262.5, 1532.1) |
1.0 (0.9, 1.2) |
Y |
|
Seroresponse % (95% CI)d |
Seroresponse % (95% CI)d |
Difference in Seroresponse Rate % (95% CI)e |
|||
|
98.9 (96.7, 99.8) |
99.3 (97.5, 99.9) |
-0.4 (-2.7, 1.5) |
|||
A descriptive efficacy analysis evaluating confirmed COVID-19 cases accrued up to the data cutoff date February 21, 2022, was performed in 5,476 participants 6 months through 5 years of age who received two doses (at 0 and 1 month) of either Moderna COVID-19 Vaccine or placebo and had a negative baseline SARS-CoV-2 status (referred to as the Per-Protocol Set for Efficacy) (for participants 6 months through 23 months, vaccine=1,511, placebo=513; for participants 2 years through 5 years, vaccine=2,594, placebo=858). For participants 6 months through 23 months in the Per-Protocol Set for Efficacy, 51.2% were male, 48.8% were female, 12.7% were Hispanic or Latino; 78.9% were White, 3.1% were African American, 4.6% were Asian, 0.2% were American Indian or Alaska Native, 0.0% were Native Hawaiian or Pacific Islander, 1.8% were other races, and 10.7% were Multiracial. For participants 2 years through 5 years, 50.7% were male, 49.3% were female, 14.0% were Hispanic or Latino, 76.8% were White, 4.1% were African American, 6.1% were Asian, 0.4% were American Indian or Alaska Native, 0.3% were Native Hawaiian or Pacific Islander, 1.6% were other races, and 10.3% were Multiracial. Between participants who received Moderna COVID-19 Vaccine and those who received placebo, there were no notable differences in demographics.
The median length of follow-up for efficacy post-Dose 2 was 68 days for participants 6 months through 23 months of age and 71 days for participants 2 years through 5 years of age.
Vaccine efficacy among individuals 6 months through 5 years in Study 4 was evaluated during the period when the B.1.1.529 (Omicron) variant was the predominant variant in circulation.
The efficacy information in individuals 6 months through 23 months of age and 2 years through 5 years of age are presented in Table 9 and Table 10, respectively.
| N = Included 15 individuals aged 2 years to 4 years randomized in the 6 months through 23 months of age group stratum (12 in the Moderna COVID-19 Vaccine group and 3 in the placebo group), and none of them had a COVID-19 case starting 14 days after Dose 2. * Vaccine efficacy defined as 1 — ratio of incidence rate (Moderna COVID-19 Vaccine vs. placebo). The 95% CI of the ratio is calculated using the exact method conditional upon the total number of cases, adjusting for person-years. a Participant must have experienced at least two of the following systemic symptoms: fever (≥38°C / ≥100.4°F), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s); or the participant must have experienced at least one of the following respiratory signs/symptoms: cough, shortness of breath or difficulty breathing, or clinical or radiographical evidence of pneumonia; and the participant must have at least one NP swab, nasal swab, or saliva sample (or respiratory sample, if hospitalized) positive for SARS-CoV-2 by RT-PCR. b Presence of at least one symptom from a list of COVID-19 symptoms and a positive NP swab or saliva sample for SARS-CoV-2 by RT-PCR. Listed symptoms were fever (temperature >38°C / ≥100.4°F), or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle aches, or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea, abdominal pain, poor appetite/poor feeding, or vomiting or diarrhea. |
|||||
|
Moderna COVID-19 Vaccine N=1,511 |
Placebo N=513 |
|
|||
|
Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
||
|
COVID-19 Cases - Definition 1a |
37 |
99.981 |
18 |
146.042 |
31.5 (-27.7, 62.0) |
|
COVID-19 Cases - Definition 2b |
51 |
138.239 |
34 |
279.822 |
50.6 (21.4, 68.6) |
| N = Included 25 individuals younger than 2 years of age randomized in the 2 years through 5 years of age group stratum (18 in the Moderna COVID-19 Vaccine group and 7 in the placebo group), and one in each treatment group had a COVID-19 case starting 14 days after Dose 2. * Vaccine efficacy defined as 1 — ratio of incidence rate (Moderna COVID-19 Vaccine vs. placebo). The 95% CI of the ratio is calculated using the exact method conditional upon the total number of cases, adjusting for person-years. a Participant must have experienced at least two of the following systemic symptoms: fever (≥38°C / ≥100.4°F), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s); or the participant must have experienced at least one of the following respiratory signs/symptoms: cough, shortness of breath or difficulty breathing, or clinical or radiographical evidence of pneumonia; and the participant must have at least one NP swab, nasal swab, or saliva sample (or respiratory sample, if hospitalized) positive for SARS-CoV-2 by RT-PCR. b Presence of at least one symptom from a list of COVID-19 symptoms and a positive NP swab or saliva sample for SARS-CoV-2 by RT-PCR. Listed symptoms were fever (temperature >38°C / ≥100.4°F), or chills, cough, shortness of breath or difficulty breathing, fatigue, muscle aches, or body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea, abdominal pain, poor appetite/poor feeding, or vomiting or diarrhea. |
|||||
|
Moderna COVID-19 Vaccine N=2,594 |
Placebo N=858 |
|
|||
|
Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
||
|
COVID-19 Cases - Definition 1a |
71 |
103.761 |
43 |
193.528 |
46.4 (19.8, 63.8) |
|
COVID-19 Cases - Definition 2b |
119 |
175.023 |
61 |
276.980 |
36.8 (12.5, 54.0) |
18.3 Immunogenicity of the Bivalent Vaccine (Original and Omicron BA.1) Administered as a Second Booster Dose
Study 5 is a Phase 2/3 open-label study in which participants 18 years of age and older, who had previously received a two-dose primary series and one booster dose of Moderna COVID-19 Vaccine, received a second booster dose with the bivalent vaccine (Original and Omicron BA.1) at least 3 months after the first booster dose. The bivalent vaccine (Original and Omicron BA.1) contained a total of 50 mcg mRNA per dose. The primary immunogenicity analysis population included 334 participants who received a booster dose of bivalent vaccine (Original and Omicron BA.1) and 260 participants who received a booster dose of Moderna COVID-19 Vaccine. Participants included in the analysis population had no serologic or virologic evidence of SARS-CoV-2 infection prior to the second booster dose.
Among participants assessed for immunogenicity, the median age of the population was 62 years (range 20-96). For the bivalent vaccine (Original and Omicron BA.1) group, 195 (58.4%) participants were age 18 to 64 and 139 (41.6%) were 65 and older; 43.4% were male, 56.6% were female, 7.2% were Hispanic or Latino, 87.1% were White, 7.2% were African American, 3.3% were Asian, 0.0% were American Indian or Alaska Native, 0.0% were Native Hawaiian or Pacific Islander, 0.6% were other races, and 1.8% were Multiracial. For the Moderna COVID-19 Vaccine group, 140 (53.8%) of participants were age 18 to 64 and 120 (46.2%) were 65 and older; 48.5% of participants were male, 51.5% were female, 8.5% were Hispanic or Latino, 90.0% were White, 4.2% were African American, 4.2% were Asian, 0.0% were American Indian or Alaska Native, 0.0% were Native Hawaiian or Pacific Islander, 0.4% were other races, and 0.0% were Multiracial. Demographic characteristics were similar among participants who received bivalent vaccine (Original and Omicron BA.1) and those who received Moderna COVID-19 Vaccine.
In Study 5, the neutralizing antibody titers (50% inhibitory dose [ID50]) against a pseudovirus expressing the original SARS-CoV-2 Spike protein (D614G) and a pseudovirus expressing the Omicron BA.1 Spike protein were evaluated. Primary immunogenicity analyses compared the ID50 GMTs and seroresponse rates (the proportion achieving a ≥4-fold rise in ID50 from pre-dose 1 of the primary series) 28 days following a second booster dose with bivalent vaccine (Original and Omicron BA.1) to those following a second booster dose with Moderna COVID-19 Vaccine. Analyses of GMTs met predefined success criteria for superiority against Omicron BA.1 and noninferiority against the Original strain. The analysis of seroresponse against Omicron BA.1 met the criterion for noninferiority: Lower limit of the 2-sided 97.5% CI for the percentage difference in seroresponse rate (bivalent vaccine [Original and Omicron BA.1] minus Moderna COVID-19 Vaccine) >-10%. Table 11 presents the analyses of ID50 GMTs; the primary analysis of seroresponse is not shown.
Post-hoc analyses evaluated the differences in seroresponse rates (the proportion achieving a ≥4-fold rise in ID50 from pre-second booster) against both the Original strain and Omicron BA.1 (Table 12).
| * Per-Protocol Immunogenicity SARS-CoV-2 Negative Set included all subjects who received the planned dose of study vaccine per schedule, had pre-booster and Day 29 neutralizing antibody data against Omicron BA.1, had no major protocol deviations that impact key or critical data, had no history of HIV infection, and had no serologic or virologic evidence of SARS-CoV-2 infection prior to the second booster dose. a The log-transformed antibody levels are analyzed using an analysis of covariance (ANCOVA) model with the treatment variable as fixed effect, adjusting for age group (<65, ≥65 years) and pre-booster antibody titer level (in log 10 scale). The treatment variable corresponds to each individual study arm dose. The resulted least square (LS) means, difference of LS means, and confidence intervals are back transformed to the original scale for presentation. b Superiority is declared if the lower limit of the 2-sided 97.5% CI for the GMT ratio is >1. c Non-inferiority is declared if the lower limit of the 2-sided 97.5% CI for the GMT ratio is ≥0.67. Note: Antibody values < the lower limit of quantitation (LLOQ) are replaced by 0.5 × LLOQ. Values > the upper limit of quantitation (ULOQ) are replaced by the ULOQ if actual values are not available. |
||||
|
|
Bivalent Vaccine (Original and Omicron BA.1) N=334 GMTa (95% CI) |
Moderna COVID-19 Vaccine N=260 GMTa (95% CI) |
GMT Ratioa (Bivalent Vaccine [Original and Omicron BA.1]/Moderna COVID-19 Vaccine) (97.5% CI) |
Met Success Criteria |
|
Omicron BA.1 |
2479.9 (2264.5, 2715.8) |
1421.2 (1283.0, 1574.4) |
1.7 (1.5, 2.0) |
Lower limit of 97.5% CI >1 Criterion: Yesb |
|
Original SARS-CoV-2 (D614G) |
6422.3 (5990.1, 6885.7) |
5286.6 (4887.1, 5718.9) |
1.2 (1.1, 1.4) |
Lower limit of 97.5% CI ≥0.67 Criterion: Yesc |
| * Per-Protocol Immunogenicity SARS-CoV-2 Negative Set included all subjects who received the planned dose of study vaccine per schedule, had pre-booster and Day 29 neutralizing antibody data against Omicron BA.1, had no major protocol deviations that impact key or critical data, had no history of HIV infection, and had no serologic or virologic evidence of SARS-CoV-2 infection prior to the second booster dose. N1=number of participants with non-missing data at pre-booster baseline and 28 days after second booster dose. n=number of participants who achieved seroresponse at 28 days after booster dose. a For post-hoc assessment of seroresponse rates, baseline was pre-second booster dose; seroresponse was defined as a change from below the LLOQ to equal or above 4 x LLOQ if participant pre-second booster dose baseline was below the LLOQ, or at least a 4-fold rise if baseline is equal to or above the LLOQ. b 95% CI is calculated using the Clopper-Pearson method. c Common risk difference and 97.5% CI is calculated using the stratified Miettinen-Nurminen method to adjust for age group (<65, ≥65 years). |
|||
|
|
Bivalent Vaccine (Original and Omicron BA.1) Seroresponsea N=334 n/N1 (%) (95% CI)b |
Moderna COVID-19 Vaccine Seroresponsea N=260 n/N1 (%) (95% CI)b |
(Bivalent Vaccine [Original and Omicron BA.1]-Moderna COVID-19 Vaccine) % (97.5% CI)c |
|
Omicron BA.1 |
250/334 (74.9) (69.8, 79.4) |
138/260 (53.1) (46.8, 59.3) |
21.6 (12.9, 30.3) |
|
Original SARS-CoV-2 (D614G) |
180/334 (53.9) (48.4, 59.3) |
111/260 (42.7) (36.6, 49.0) |
11.2 (2.1, 20.3) |
18.4 Immunogenicity of Moderna COVID-19 Vaccine Administered as First Booster Dose Following a Primary Series of Moderna COVID-19 Vaccine in Participants 18 Years of Age and Older
Effectiveness of a booster dose of the Moderna COVID-19 Vaccine was based on assessment of neutralizing antibody titers (ID50) against a pseudovirus expressing the SARS-CoV-2 Spike protein from a USA_WA1/2020 isolate carrying the D614G mutation. Immunogenicity analyses compared the ID50 following the booster dose to the ID50 following the primary series.
In an open-label phase of Study 2, participants 18 years of age and older received a single booster dose (50 mcg mRNA; 0.25 mL) at least 6 months after completion of the primary series. The primary immunogenicity analysis population included 149 booster dose participants in Study 2 (including one individual who had only received a single dose of the primary series) and a random subset of 1,055 participants from Study 1 who had completed primary vaccination with Moderna COVID-19 Vaccine. Study 1 and 2 participants included in the analysis population had no serologic or virologic evidence of SARS-CoV-2 infection prior to the first primary series dose and prior to the booster dose, respectively. Among participants assessed for immunogenicity, 60.4% were female, 6.7% were Hispanic or Latino; 95.3% were White, 3.4% were Black or African American, 0.7% were Asian, and 0.7% were American Indian or Alaskan Native; 9.4% were obese (body mass index ≥30 kg/m2). The median age of Study 2 participants was 56 years of age (range 18-82) and 24.8% of participants were 65 years of age and older. Study 2 participants included in the primary immunogenicity analysis population did not have pre-existing medical conditions that would place them at risk of severe COVID-19. Study 1 participants included in the primary immunogenicity analysis population were a stratified random sample which reflected the overall primary efficacy analysis population with regards to demographics and pre-existing medical conditions with a higher percentage of those ≥65 years of age (33.6%), with risk factors for severe COVID-19 (39.4%), and communities of color (53.5%).
Immunogenicity analyses included an assessment of ID50 geometric mean titer (GMT) ratio and difference in seroresponse rates. The analysis of the GMT ratio of ID50 following the booster dose compared to the primary series met the immunobridging criteria for a booster response. Seroresponse for a participant was defined as achieving a ≥4-fold rise in ID50 from baseline (before the booster dose in Study 2 and before the first dose of the primary series in Study 1). The lower limit of the 2-sided 95% CI for the difference in seroresponse rates between Study 1 and Study 2 was -16.7%, which did not meet the immunobridging criterion for a booster response (lower limit of 2-sided 95% CI for the percentage difference of ≥ -10%). These analyses are summarized in Table 13 and Table 14.
| * Per-Protocol Immunogenicity Set included all subjects who had both baseline (or Study 2 Day 1 for Study 2) and post-vaccination immunogenicity samples, did not have SARS-CoV-2 infection at baseline (or Study 2 Day 1 for Study 2), did not have a major protocol deviation that impacted immune response, and had post-injection immunogenicity assessment at timepoint of primary interest (Day 29 for Study 2 and Day 57 for Study 1). a Number of subjects with non-missing data at the corresponding timepoint. b Given the lack of randomization in Study 2, the statistical analysis plan pre-specified an analysis of covariance model for estimating the geometric mean titer that adjusts for differences in age groups (<65 years, ≥65 years). c Immunobridging is declared if the lower limit of the 2-sided 95% CI for the GMR is >0.67 and the point estimate of the GLSM ratio is ≥1.0. Note: Antibody values < the lower limit of quantitation (LLOQ) are replaced by 0.5 × LLOQ. Values > the upper limit of quantitation (ULOQ) are replaced by the ULOQ if actual values are not available. GLSM = Geometric least squares mean GMR = Geometric mean ratio |
|||
|
Study 2 Booster Dose Na=149 GMTb (95% CI) |
Study 1 Primary Series Na=1053 GMTb (95% CI) |
GMT Ratio (Study 2/Study 1) |
Met Success Criteriac |
|
1802 (1548, 2099) |
1027 (968, 1089) |
1.8 (1.5, 2.1) |
Lower limit of 95% CI ≥0.67 Criterion: Yes Point Estimate ≥1.0 Criterion: Yes |
| * Per-Protocol Immunogenicity Set included all subjects who had both baseline (or Study 2 Day 1 for Study 2) and post-vaccination immunogenicity samples, did not have SARS-CoV-2 infection at baseline (or Study 2 Day 1 for Study 2), did not have a major protocol deviation that impacted immune response, and had post-injection immunogenicity assessment at timepoint of primary interest (Day 29 for Study 2 and Day 57 for Study 1). a Seroresponse is defined as ≥4-fold rise of pseudovirus neutralizing antibody titers (ID50) from baseline (pre-booster dose in Study 2 and pre-Dose 1 in Study 1), where baseline titers < LLOQ are set to LLOQ for the analysis. b Number of subjects with non-missing data at both baseline and the post-baseline timepoint of interest. c 95% CI is calculated using the Clopper-Pearson method. d 95% CI is calculated using the Miettinen-Nurminen (score) confidence limits. e Immunobridging is declared if the lower limit of the 2-sided 95% CI for the percentage difference is > -10%. |
|||
|
Study 2 Booster Seroresponsea Nb=149 n (%) (95% CI)c |
Study 1 Primary Series Seroresponsea Nb=1050 n (%) (95% CI)c |
Difference in Seroresponse Rate (Study 2-Study 1) % (95% CI)d |
Met Success Criterione |
|
131 (87.9) (81.6, 92.7) |
1033 (98.4) (97.4, 99.1) |
-10.5 (-16.7, -6.1) |
Lower limit of 95% CI ≥-10% Criterion: No |
Study 2 participants who met the ≥4-fold increase in titer post-booster dose (87.9%) had a lower baseline GMT of 109 (range of individual titers 9, 4393), whereas Study 2 participants who did not meet the ≥4-fold increase in titers post-booster had a higher baseline GMT of 492 (range of individual titers 162, 2239).
An additional descriptive analysis evaluated seroresponse rates using baseline neutralizing antibody titers prior to Dose 1 of the primary series. As shown in Table 15 below, the booster dose seroresponse rate, with seroresponse defined as at least a 4-fold rise relative to the pre-Dose 1 titer, was 100%. The difference in seroresponse rates in this post-hoc analysis was 1.6% (95% CI -0.9, 2.6).
| * Per-Protocol Immunogenicity Set included all subjects who had non-missing data at baseline (before Dose 1) and 28 days post-booster in Study 2 or 28 days post-Dose 2 in the primary series in Study 1, respectively, did not have SARS-CoV-2 infection at pre-booster in Study 2 or baseline in Study 1, did not have a major protocol deviation that impacted immune response, and had post-injection immunogenicity assessment at timepoint of primary interest. a Seroresponse is defined as ≥4-fold rise of pseudovirus neutralizing antibody titers (ID50) from pre-Dose 1, where baseline titers < LLOQ are set to LLOQ for the analysis. b Number of subjects with non-missing data at baseline (before Dose 1) and 28 days post-booster in Study 2. c Number of subjects with non-missing data at baseline (before Dose 1) and 28 days post-Dose 2 in the primary series in Study 1. d 95% CI is calculated using the Clopper-Pearson method. e 95% CI is calculated using the Miettinen-Nurminen (score) confidence limits. |
||
|
Study 2 Booster Seroresponsea Nb=148 n (%) (95% CI)d |
Study 1 Primary Series Seroresponsea Nc=1050 n (%) (95% CI)d |
(After Booster-After Primary Series) % (95% CI)e |
|
148 (100) (97.5, 100) |
1033 (98.4) (97.4, 99.1) |
1.6 (-0.9, 2.6) |
18.5 Immunogenicity of Moderna COVID-19 Vaccine Booster Dose Following Moderna COVID-19 Vaccine Primary Series in Participants 17 Months Through 5 Years of Age
Effectiveness of a booster dose of the Moderna COVID-19 Vaccine in individuals 6 months through 5 years of age is based on a comparison of immune responses, as assessed by neutralizing antibody concentration against a pseudovirus expressing the SARS-CoV-2 Spike protein from a USA_WA1/2020 isolate carrying the D614G mutation, following the booster dose in study participants 17 months through 5 years of age to that following the primary series in adults 18 through 25 years.
In an open-label phase of Study 4, participants 17 months through 5 years of age received a single booster dose of Moderna COVID-19 Vaccine (10 mcg mRNA) at least 6 months after completion of a Moderna COVID-19 Vaccine primary series (two doses 1 month apart). The primary immunogenicity analysis population included 56 booster dose participants in Study 4 and a random subset of 295 participants 18 through 25 years from Study 1 who had completed primary vaccination with two doses of Moderna COVID-19 Vaccine (100 mcg mRNA per dose) 1 month apart. Study 1 and 4 participants included in the analysis population had no serologic or virologic evidence of SARS-CoV-2 infection prior to the first primary series dose and prior to the booster dose, respectively. Among participants 17 months through 5 years assessed for immunogenicity, 50.0% were male, 50.0% were female, 7.1% were Hispanic or Latino; 78.6% were White, 1.8% were Black or African American, 7.1% were Asian, 0.0% were American Indian or Alaskan Native, 0.0% were Native Hawaiian or Pacific Islander, 3.6% were other races, and 8.9% were Multiracial. Among the 56 participants in the primary immunogenicity analysis population, the median age for receipt of the booster dose was 2.3 years (range 1.4-5.6 years).
The primary immunogenicity analyses of the GMC ratio and difference in seroresponse rates following the booster dose in Study 4 compared to following the primary series in Study 1 met the pre-defined immunobridging success criteria. Seroresponse for a participant was defined as achieving a ≥4-fold rise of neutralizing antibody concentration from baseline (before the first dose of the primary series in Study 4 and Study 1). These analyses are summarized in Table 16.
| * Per-Protocol Immunogenicity Subset – Pre-Booster SARS-CoV-2 Negative for Study 4 included all subjects who had both pre-booster and post-booster immunogenicity samples, did not have SARS-CoV-2 infection at pre-booster, did not have a major protocol deviation that impacted immune response, and had post-booster immunogenicity assessment at timepoint of primary interest (28 days post-Booster Dose). † Per-Protocol Immunogenicity Subset for Study 1 included all subjects who had both baseline (pre-vaccination) and post-vaccination immunogenicity samples, did not have SARS-CoV-2 infection at baseline, did not have a major protocol deviation that impacted immune response, and had post-injection immunogenicity assessment at timepoint of primary interest (28 days post-Dose 2). a Number of subjects with non-missing data at the corresponding timepoint. b Immunobridging success is declared if the lower limit of the 2-sided 95% CI for the GMC Ratio is ≥0.667. c Seroresponse is defined as ≥4-fold rise of pseudovirus neutralizing antibody concentration from baseline (pre-Dose 1 of primary series in Study 4 and Study 1), where baseline concentration < LLOQ is set to LLOQ for the analysis. N1=number of participants with non-missing data at pre-vaccination baseline and 28 days post-Booster Dose for Study 4 or 28 days post-Dose 2 for Study 1. n=number of participants who achieved seroresponse at 28 days post-Booster Dose for Study 4 or 28 days post-Dose 2 for Study 1. d 95% CI is calculated using the Clopper-Pearson method. e 95% CI is calculated using the Miettinen-Nurminen (score) confidence limits. f Immunobridging success is declared if the lower limit of the 2-sided 95% CI for the percentage difference is ≥ -10%. Note: Antibody values < the lower limit of quantitation (LLOQ) are replaced by 0.5 × LLOQ. Values > the upper limit of quantitation (ULOQ) are replaced by the ULOQ if actual values are not available. |
|||
|
Study 4* Booster Dose Na=56 GMC (95% CI) |
Study 1† Primary Series Na=294 GMC (95% CI) |
GMC Ratio (Study 4/Study 1) |
Met Success Criterion |
|
5713 (4604, 7089) |
1400 (1275, 1539) |
4.1 (3.2, 5.2) |
Yesb |
|
Study 4 Booster Dose Seroresponsec N=56 n/N1 (%) (95% CI)d |
Study 1 Primary Series Seroresponsec N=294 n/N1 (%) (95% CI)d |
Difference in Seroresponse Rate (Study 4-Study 1) % (95% CI)e |
|
|
53/53 (100) (93.3, 100.0) |
292/294 (99.3) (97.6, 99.9) |
0.7 (-6.1, 2.4) |
Yesf |
In a descriptive analysis, the booster dose seroresponse rate among participants 17 months through 5 years of age, with seroresponse defined as at least a 4-fold rise relative to the pre-booster concentration, was 94.6%. The difference in seroresponse rates (Study 4 participants minus Study 1 participants) in this post-hoc analysis was -4.7% (95% CI -14.0, -0.9).
19 HOW SUPPLIED/STORAGE AND HANDLING
The information in this section applies to Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) that is supplied in multiple-dose vials with dark pink caps and labels with a yellow box. Each multiple-dose vial contains 2 doses of 0.2 mL. These multiple-dose vials are supplied as follows:
NDC: 80777-283-99 Carton of 10 multiple-dose vials
NDC: 80777-283-02 Multiple-dose vial containing 2 doses of 0.2 mL
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Frozen Storage
Store frozen between -50°C to -15°C (-58°F to 5°F).
Storage after Thawing
-
Storage at 2°C to 8°C (36°F to 46°F):
- o Vials may be stored refrigerated between 2°C to 8°C (36°F to 46°F) for up to 30 days prior to first use, provided the expiration date is not exceeded.
- o Vials should be discarded 8 hours after the first puncture.
-
Storage at 8°C to 25°C (46°F to 77°F):
- o Vials may be stored between 8°C to 25°C (46°F to 77°F) for a total of 24 hours.
- o Vials should be discarded 8 hours after the first puncture.
- o Total storage at 8°C to 25°C (46°F to 77°F) must not exceed 24 hours.
Do not refreeze once thawed.
Thawed vials can be handled in room light conditions.
Transportation of Thawed Vials at 2°C to 8°C (36°F to 46°F)
If transport at -50°C to -15°C (-58°F to 5°F) is not feasible, available data support transportation of one or more thawed vials for up to 12 hours at 2°C to 8°C (36°F to 46°F) when shipped using shipping containers which have been qualified to maintain 2°C to 8°C (36°F to 46°F) and under routine road and air transport conditions with shaking and vibration minimized. Once thawed and transported at 2°C to 8°C (36°F to 46°F), vials should not be refrozen and should be stored at 2°C to 8°C (36°F to 46°F) until use.
20 PATIENT COUNSELING INFORMATION
Advise the recipient or caregiver to read the “FACT SHEET FOR RECIPIENTS AND CAREGIVERS.”
The vaccination provider must include vaccination information in the state/local jurisdiction’s Immunization Information System (IIS) or other designated system. Advise recipient or caregiver that more information about IISs can be found at: https://www.cdc.gov/vaccines/programs/iis/about.html.
21 CONTACT INFORMATION
For general questions, send an email or call the telephone number provided below.
|
|
Telephone number |
|
1-866-MODERNA (1-866-663-3762) |
This EUA Prescribing Information may have been updated. For the most recent Full EUA Prescribing Information, please visit www.modernatx.com/covid19vaccine-eua.
Moderna US, Inc.
Cambridge, MA 02139
©2022 ModernaTX, Inc. All rights reserved.
Patent(s): www.modernatx.com/patents
Revised: Dec/8/2022
FACT SHEET FOR RECIPIENTS AND CAREGIVERS
EMERGENCY USE AUTHORIZATION OF MODERNA COVID-19 VACCINE AND MODERNA COVID-19 VACCINE, BIVALENT (ORIGINAL AND OMICRON BA.4/BA.5) TO PREVENT CORONAVIRUS DISEASE 2019 (COVID-19)
|
|
Your child is being offered either Moderna COVID-19 Vaccine or Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), hereafter referred to as Moderna COVID-19 Vaccine, Bivalent, to prevent Coronavirus Disease 2019 (COVID-19) caused by SARS-CoV-2.
This Fact Sheet for Recipients and Caregivers comprises the Fact Sheet for the authorized Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent for use in individuals 6 months through 5 years of age.5
Moderna COVID-19 Vaccine has received Emergency Use Authorization (EUA) from FDA to provide:
- a two-dose primary series to individuals 6 months through 5 years of age; and
- a third primary series dose to individuals 6 months through 5 years of age with certain kinds of immunocompromise.
Moderna COVID-19 Vaccine, Bivalent has received EUA from FDA to provide a single booster dose to individuals 6 months through 5 years of age at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine.
Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent are not FDA-approved for use in individuals 6 months through 5 years of age.
This Fact Sheet contains information to help you understand the risks and benefits of Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent, which your child may receive because there is currently a pandemic of COVID-19. Talk to your child's vaccination provider if you have questions.
Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent may not protect everyone.
This Fact Sheet may have been updated. For the most recent Fact Sheet, please visit www.modernatx.com/covid19vaccine-eua.
WHAT YOU NEED TO KNOW BEFORE YOUR CHILD GETS THIS VACCINE
WHAT IS COVID-19?
COVID-19 is caused by a coronavirus called SARS-CoV-2. This type of coronavirus has not been seen before. You can get COVID-19 through contact with another person who has the virus. It is predominantly a respiratory illness that can affect other organs. People with COVID-19 have had a wide range of symptoms reported, ranging from mild symptoms to severe illness. Symptoms may appear 2 to 14 days after exposure to the virus. Symptoms may include: fever or chills; cough; shortness of breath; fatigue; muscle or body aches; headache; new loss of taste or smell; sore throat; congestion or runny nose; nausea or vomiting; diarrhea.
HOW ARE MODERNA COVID-19 VACCINE AND MODERNA COVID-19 VACCINE, BIVALENT RELATED?
Moderna COVID-19 Vaccine, Bivalent is made in the same way as Moderna COVID-19 Vaccine, but it also contains an Omicron component to help prevent COVID-19 caused by the Omicron variant of SARS-CoV-2.
For more information on EUA, see the “What is an Emergency Use Authorization (EUA)?” section at the end of this Fact Sheet.
WHAT SHOULD YOU MENTION TO YOUR CHILD’S VACCINATION PROVIDER BEFORE YOUR CHILD GETS EITHER OF THESE VACCINES?
Tell the vaccination provider about all of your child’s medical conditions, including if your child:
- has any allergies
- has had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart)
- has a fever
- has a bleeding disorder or is on a blood thinner
- is immunocompromised or is on a medicine that affects your child’s immune system
- has received another COVID-19 vaccine
- has ever fainted in association with an injection
WHO SHOULD NOT GET MODERNA COVID-19 VACCINE OR MODERNA COVID-19 VACCINE, BIVALENT?
Your child should not get either of these vaccines if your child:
- had a severe allergic reaction after a previous dose of Moderna COVID-19 Vaccine
- had a severe allergic reaction to any ingredient of these vaccines
WHAT ARE THE INGREDIENTS IN THESE VACCINES?
Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent contain the following ingredients: messenger ribonucleic acid (mRNA), lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate trihydrate, and sucrose.
HOW ARE THESE VACCINES GIVEN?
Moderna COVID-19 Vaccine or Moderna COVID-19 Vaccine, Bivalent will be given to your child as an injection into the muscle.
Primary Series: Moderna COVID-19 Vaccine is administered as a two-dose series, 1 month apart. A third primary series dose may be administered at least 1 month after the second dose to individuals who are determined to have certain kinds of immunocompromise.
Booster Dose: Moderna COVID-19 Vaccine, Bivalent is administered as a single booster dose at least 2 months after completion of primary vaccination with Moderna COVID-19 Vaccine.
HAVE THESE VACCINES BEEN USED BEFORE?
Millions of individuals 6 months of age and older have received the Moderna COVID-19 Vaccine under EUA. In a clinical trial, approximately 5,000 individuals 6 months through 5 years of age have received at least 1 dose of Moderna COVID-19 Vaccine. In other clinical trials, approximately 4,000 individuals 6 years through 11 years of age and 30,000 individuals 12 years of age and older have received at least 1 dose of Moderna COVID-19 Vaccine.
Millions of individuals 6 years of age and older have received Moderna COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) under EUA. In a clinical trial, approximately 400 individuals 18 years of age and older received 1 dose of a bivalent vaccine that differs from the Moderna COVID-19 Vaccine, Bivalent in that it contains a different Omicron component.
WHAT ARE THE BENEFITS OF THESE VACCINES?
Moderna COVID-19 Vaccine has been shown to prevent COVID-19. FDA has authorized Moderna COVID-19 Vaccine, Bivalent to provide better protection against COVID-19 caused by the Omicron variant of SARS-CoV-2.
The duration of protection against COVID-19 is currently unknown.
WHAT ARE THE RISKS OF THESE VACCINES?
There is a remote chance that the vaccine could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose. For this reason, your child’s vaccination provider may ask your child to stay at the place where your child received the vaccine for monitoring after vaccination. Signs of a severe allergic reaction can include:
- Difficulty breathing
- Swelling of your face and throat
- A fast heartbeat
- A bad rash all over your body
- Dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received Moderna COVID-19 Vaccine and Moderna COVID-19 Vaccine, Bivalent. In most of these people, symptoms began within a few days following vaccination. The chance of having this occur is very low. You should seek medical attention right away if your child has any of the following symptoms after receiving the vaccine, particularly during the 2 weeks after your child receives a dose of the vaccine:
- Chest pain
- Shortness of breath or difficulty breathing
- Feelings of having a fast-beating, fluttering, or pounding heart
- Fainting
- Unusual and persistent irritability
- Unusual and persistent poor feeding
- Unusual and persistent fatigue or lack of energy
- Persistent vomiting
- Persistent pain in the abdomen
- Unusual and persistent cool, pale skin
Side effects that have been reported in clinical trials with these vaccines include:
- Injection site reactions: pain, tenderness and swelling of the lymph nodes in the same arm of the injection or in the groin, redness, and swelling (hardness)
- General side effects: fatigue, headache, muscle pain, chills, nausea and vomiting, fever, joint pain, irritability/crying, sleepiness, and loss of appetite
Side effects that have been reported during post-authorization use include:
- Severe allergic reactions
- Urticaria (itchy rash/hives)
- Myocarditis (inflammation of the heart muscle)
- Pericarditis (inflammation of the lining outside the heart)
- Fainting in association with injection of the vaccine
These may not be all the possible side effects of these vaccines. Serious and unexpected side effects may occur. The possible side effects of these vaccines are still being studied.
WHAT SHOULD I DO ABOUT SIDE EFFECTS?
If your child experiences a severe allergic reaction, call 9-1-1, or go to the nearest hospital.
Call the vaccination provider or your child’s healthcare provider if your child has any side effects that bother your child or do not go away.
Report vaccine side effects to FDA/CDC Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to https://vaers.hhs.gov/reportevent.html. Please include “Moderna COVID-19 Vaccine EUA” or “Moderna COVID-19 Vaccine, Bivalent EUA”, as appropriate, in the first line of box #18 of the report form.
In addition, you can report side effects to ModernaTX, Inc. at 1-866-MODERNA (1-866-663-3762).
You may also be given an option to enroll in v-safe. V-safe is a voluntary smartphone-based tool that uses text messaging and web surveys to check in with people who have been vaccinated to identify potential side effects after COVID-19 vaccination. V-safe asks questions that help CDC monitor the safety of COVID-19 vaccines. V-safe also provides dose reminders if needed and live telephone follow-up by CDC if participants report a significant health impact following COVID-19 vaccination. For more information on how to sign up, visit: www.cdc.gov/vsafe.
WHAT IF I DECIDE NOT TO HAVE MY CHILD GET MODERNA COVID-19 VACCINE OR MODERNA COVID-19 VACCINE, BIVALENT?
Under the EUA, there is an option to accept or refuse receiving the vaccine. Should you decide for your child not to receive either of these vaccines, it will not change your child's standard medical care.
ARE OTHER CHOICES AVAILABLE FOR PREVENTING COVID-19 BESIDES MODERNA COVID-19 VACCINE OR MODERNA COVID-19 VACCINE, BIVALENT?
For primary vaccination for children 6 months through 5 years of age, other vaccines to prevent COVID-19 may be available under EUA. For booster vaccination for children 5 years of age who have completed primary vaccination with an FDA authorized COVID-19 vaccine, other bivalent vaccines that contain an Omicron component of SARS-CoV-2 may be available under EUA. For booster vaccination for children 6 months through 4 years of age who have completed primary vaccination with Moderna COVID-19 Vaccine, the Moderna COVID-19 Vaccine, Bivalent is the only vaccine available under EUA.
CAN MY CHILD RECEIVE MODERNA COVID-19 VACCINE OR MODERNA COVID-19 VACCINE, BIVALENT AT THE SAME TIME AS OTHER VACCINES?
Data have not yet been submitted to FDA on administration of Moderna COVID-19 Vaccine or Moderna COVID-19 Vaccine, Bivalent at the same time as other vaccines. If you are considering having your child receive Moderna COVID-19 Vaccine or Moderna COVID-19 Vaccine, Bivalent with other vaccines, discuss your options with your child’s healthcare provider.
WHAT IF MY CHILD IS IMMUNOCOMPROMISED?
If your child is immunocompromised, you may be given the option to have your child receive a third primary series dose of Moderna COVID-19 Vaccine. Immunocompromised children 6 months through 5 years of age who have completed primary vaccination with Moderna COVID-19 Vaccine may receive a single booster dose with the Moderna COVID-19 Vaccine, Bivalent. Vaccinations may not provide full immunity to COVID-19 in people who are immunocompromised, and you should continue to have your child maintain physical precautions to help prevent COVID-19. Your child’s close contacts should be vaccinated as appropriate.
WILL THESE VACCINES GIVE MY CHILD COVID-19?
No. These vaccines do not contain SARS-CoV-2 and cannot give your child COVID-19.
KEEP YOUR CHILD’S VACCINATION CARD
When your child gets the first COVID-19 vaccine, you will get a vaccination card. Remember to bring the card when you return.
ADDITIONAL INFORMATION
If you have questions, visit the website or call the telephone number provided below.
To access the most recent Fact Sheets, please scan the QR code provided below.
|
Moderna COVID-19 Vaccine website |
Telephone number |
|
www.modernatx.com/covid19vaccine-eua  |
1-866-MODERNA (1-866-663-3762) |
HOW CAN I LEARN MORE?
- Ask the vaccination provider
- Visit CDC at https://www.cdc.gov/coronavirus/2019-ncov/index.html
- Visit FDA at https://www.fda.gov/emergency-preparedness-and-response/mcm-legal- regulatory-and-policy-framework/emergency-use-authorization
- Contact your state or local public health department
WHERE WILL MY CHILD’S VACCINATION INFORMATION BE RECORDED?
The vaccination provider may include your child’s vaccination information in your state/local jurisdiction’s Immunization Information System (IIS) or other designated system. For more information about IISs, visit: https://www.cdc.gov/vaccines/programs/iis/about.html.
CAN I BE CHARGED AN ADMINISTRATION FEE FOR RECEIPT OF THESE COVID-19 VACCINES?
No. At this time, the provider cannot charge you for a vaccine dose and you cannot be charged an out-of-pocket vaccine administration fee or any other fee if only receiving a COVID-19 vaccination. However, vaccination providers may seek appropriate reimbursement from a program or plan that covers COVID-19 vaccine administration fees for the vaccine recipient (private insurance, Medicare, Medicaid, HRSA COVID-19 Uninsured Program for non-insured recipients).
WHERE CAN I REPORT CASES OF SUSPECTED FRAUD?
Individuals becoming aware of any potential violations of the CDC COVID-19 Vaccination Program requirements are encouraged to report them to the Office of the Inspector General, U.S. Department of Health and Human Services, at 1-800-HHS-TIPS or TIPS.HHS.GOV.
WHAT IS THE COUNTERMEASURES INJURY COMPENSATION PROGRAM?
The Countermeasures Injury Compensation Program (CICP) is a federal program that may help pay for costs of medical care and other specific expenses of certain people who have been seriously injured by certain medicines or vaccines, including these vaccines. Generally, a claim must be submitted to the CICP within one (1) year from the date of receiving the vaccine. To learn more about this program, visit www.hrsa.gov/cicp/ or call 1-855-266-2427.
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
An EUA is a mechanism to facilitate the availability and use of medical products, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. An EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic. A product authorized for emergency use has not undergone the same type of review by FDA as an FDA approved product.
FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of the scientific evidence available showing that the product may be effective to prevent COVID-19 during the COVID-19 pandemic and that the known and potential benefits of the product outweigh the known and potential risks of the product. All of these criteria must be met to allow for the product to be used during the COVID-19 pandemic.
An EUA is in effect for the duration of the COVID-19 EUA declaration justifying emergency use of this product, unless terminated or revoked (after which the product may no longer be used).
Moderna US, Inc.
Cambridge, MA 02139
©2022 ModernaTX, Inc. All rights reserved.
Patent(s): www.modernatx.com/patents
Revised: Dec/8/2022
NDC: 80777-283-02
Moderna COVID-19 Vaccine, Bivalent
Original and Omicron BA.4/BA.5
BOOSTER DOSES ONLY
Age 6m through 5y
For IM Use. For use under EUA.
Vial contains 2 doses of 0.2 mL
Scan carton QR code for expiry date
Package/Label Display Panel
NDC: 80777-283-99
Moderna COVID-19 Vaccine, Bivalent
Suspension for Intramuscular Injection
Original and Omicron BA.4/BA.5
For use under Emergency Use Authorization
BOOSTER DOSES ONLY
For age 6 months through 5 years
STORE FROZEN between -50° to -15°C (-58° to 5°F).
Protect from light
Ten multi-dose vials
Each vial contains 2 doses of 0.2 m
| MODERNA COVID-19 VACCINE, BIVALENT
moderna covid-19 vaccine, bivalent injection, suspension |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Moderna US, Inc. (117626450) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ModernaTX, Inc. | 116912313 | ANALYSIS(80777-283) , API MANUFACTURE(80777-283) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Indiana, LLC | 172209277 | MANUFACTURE(80777-283) , ANALYSIS(80777-283) , LABEL(80777-283) , PACK(80777-283) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.