THEOPHYLLINE (ANHYDROUS)- theophylline anhydrous tablet, extended release
Theophylline by
Drug Labeling and Warnings
Theophylline by is a Prescription medication manufactured, distributed, or labeled by Westminster Pharmaceuticals, LLC, MPP Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Theophylline (Anhydrous) Extended-Release Tablets in a controlled-release system allows a 24-hour dosing interval for appropriate patients.
Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione,3,7-dihydro-1,3-dimethyl-, and is represented by the following structural formula:
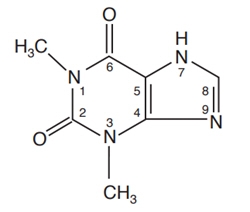
The molecular formula of anhydrous theophylline is C7H8N4O2 with a molecular weight of 180.17.
Each extended-release tablet for oral administration, contains 400 or 600 mg of anhydrous theophylline.
Inactive ingredients: glyceryl behenate, silicified microcrystalline cellulose, silicon dioxide, and magnesium stearate.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Theophylline has two distinct actions in the airways of patients with reversible obstruction; smooth muscle relaxation (i.e., bronchodilation) and suppression of the response of the airways to stimuli (i.e., non-bronchodilator prophylactic effects). While the mechanisms of action of theophylline are not known with certainty, studies in animals suggest that bronchodilation is mediated by the inhibition of two isozymes of phosphodiesterase (PDE III and, to a lesser extent, PDE IV) while non-bronchodilator prophylactic actions are probably mediated through one or more different molecular mechanisms, that do not involve inhibition of PDE III or antagonism of adenosine receptors. Some of the adverse effects associated with theophylline appear to be mediated by inhibition of PDE III (e.g., hypotension, tachycardia, headache, and emesis) and adenosine receptor antagonism (e.g., alterations in cerebral blood flow).
Theophylline increases the force of contraction of diaphragmatic muscles. This action appears to be due to enhancement of calcium uptake through an adenosine-mediated channel.
Serum Concentration-Effect Relationship
Bronchodilation occurs over the serum theophylline concentration range of 5-20 mcg/mL. Clinically important improvement in symptom control has been found in most studies to require peak serum theophylline concentrations >10 mcg/mL, but patients with mild disease may benefit from lower concentrations. At serum theophylline concentrations >20 mcg/mL, both the frequency and severity of adverse reactions increase. In general, maintaining peak serum theophylline concentrations between 10 and 15 mcg/mL will achieve most of the drug's potential therapeutic benefit while minimizing the risk of serious adverse events.
Pharmacokinetics
Overview
Theophylline is rapidly and completely absorbed after oral administration in solution or immediate-release solid oral dosage form. Theophylline does not undergo any appreciable pre-systemic elimination, distributes freely into fat-free tissues and is extensively metabolized in the liver.
The pharmacokinetics of theophylline vary widely among similar patients and cannot be predicted by age, sex, body weight or other demographic characteristics. In addition, certain concurrent illnesses and alterations in normal physiology (See Table I) and co-administration of other drugs (See Table II) can significantly alter the pharmacokinetic characteristics of theophylline. Within-subject variability in metabolism has also been reported in some studies, especially in acutely ill patients. It is, therefore, recommended that serum theophylline concentrations be measured frequently in acutely ill patients (e.g., at 24-hr intervals) and periodically in patients receiving long-term therapy, e.g., at 6-12 month intervals. More frequent measurements should be made in the presence of any condition that may significantly alter theophylline clearance (see PRECAUTIONS, Laboratory Tests).
Table I. Mean and range of total body clearance and half-life of theophylline related to age and altered physiological states.* Population Characteristics Total body clearance†
mean (range) ‡
(mL/kg/min)Half-life
mean (range)‡
(hr)- * For various North American patient populations from literature reports. Different rates of elimination and consequent dosage requirements have been observed among other peoples.
- † Clearance represents the volume of blood completely cleared of theophylline by the liver in one minute. Values listed were generally determined at serum theophylline concentrations <20 mcg/mL; clearance may decrease and half-life may increase at higher serum concentrations due to non-linear pharmacokinetics.
- ‡ Reported range or estimated range (mean±2 SD) where actual range not reported.
- § NR=not reported or not reported in a comparable format.
- ¶ Median
Age Premature neonates postnatal age 3-15 days 0.29 (0.09-0.49) 30 (17-43) postnatal age 25-57 days 0.64 (0.04-1.2) 20 (9.4-30.6) Term infants postnatal age 1-2 days NR§ 25.7 (25-26.5) postnatal age 3-30 weeks NR§ 11 (6-29) Children 1-4 years 1.7 (0.5-2.9) 3.4 (1.2-5.6) 4-12 years 1.6 (0.8-2.4) NR§ 13-15 years 0.9 (0.48-1.3) NR§ 6-17 years 1.4 (0.2-2.6) 3.7 (1.5-5.9) Adults (16-60 years) otherwise healthy non-smoking asthmatics 0.65 (0.27-1.03) 8.7 (6.1-12.8) Elderly (>60 years) non-smokers with normal cardiac, liver, and renal function 0.41 (0.21-0.61) 9.8 (1.6-18) Concurrent illness or altered physiological state Acute pulmonary edema 0.33¶ (0.07-2.45) 19¶ (3.1-82) COPD->60 years, stable non-smoker >1 year 0.54 (0.44-0.64) 11 (9.4-12.6) COPD with cor pulmonale 0.48 (0.08-0.88) NR§ Cystic fibrosis (14-28 years) 1.25 (0.31-2.2) 6.0 (1.8-10.2) Fever associated with acute viral respiratory illness (children 9-15 years) NR§ 7.0 (1.0-13) Liver disease cirrhosis 0.31¶ (0.1-0.7) 32¶ (10-56) acute hepatitis 0.35 (0.25-0.45) 19.2 (16.6-21.8) cholestasis 0.65 (0.25-1.45) 14.4 (5.7-31.8) Pregnancy 1st trimester NR§ 8.5 (3.1-13.9) 2nd trimester NR§ 8.8 (3.8-13.8) 3rd trimester NR§ 13.0 (8.4-17.6) Sepsis with multi-organ failure 0.47 (0.19-1.9) 18.8 (6.3-24.1) Thyroid disease hypothyroid 0.38 (0.13-0.57) 11.6 (8.2-25) hyperthyroid 0.8 (0.68-0.97) 4.5 (3.7-5.6) Note: In addition to the factors listed above, theophylline clearance is increased and half-life decreased by low carbohydrate/high protein diets, parenteral nutrition, and daily consumption of charcoal-broiled beef. A high carbohydrate/low protein diet can decrease the clearance and prolong the half-life of theophylline.
Absorption
Theophylline (Anhydrous) Extended-Release Tablets administered in the fed state is completely absorbed after oral administration.
In a single-dose crossover study, two 400 mg Theophylline (Anhydrous) Extended-Release Tablets were administered to 19 normal volunteers in the morning or evening immediately following the same standardized meal (769 calories consisting of 97 grams carbohydrates, 33 grams protein and 27 grams fat). There was no evidence of dose dumping nor were there any significant differences in pharmacokinetic parameters attributable to time of drug administration. On the morning arm, the pharmacokinetic parameters were AUC=241.9±83.0 mcg hr/mL, Cmax=9.3±2.0 mcg/mL, Tmax=12.8±4.2 hours. On the evening arm, the pharmacokinetic parameters were AUC=219.7± 83.0 mcg hr/mL, Cmax=9.2±2.0 mcg/mL, Tmax=12.5±4.2 hours.
A study in which Theophylline (Anhydrous) Extended-Release 400 mg Tablets were administered to 17 fed adult asthmatics produced similar theophylline level-time curves when administered in the morning or evening. Serum levels were generally higher in the evening regimen but there were no statistically significant differences between the two regimens.
MORNING EVENING AUC (0-24 hrs) (mcg hr/mL) 236.0±76.7 256.0±80.4 Cmax (mcg/mL) 14.5±4.1 16.3±4.5 Cmin (mcg/mL) 5.5±2.9 5.0±2.5 Tmax (hours) 8.1±3.7 10.1±4.1 A single-dose study in 15 normal fasting male volunteers whose theophylline inherent mean elimination half-life was verified by a liquid theophylline product to be 6.9±2.5 (SD) hours were administered two or three 400 mg Theophylline (Anhydrous) Extended-Release Tablets. The relative bioavailability of Theophylline (Anhydrous) Extended-Release Tablets given in the fasting state in comparison to an immediate-release product was 59%. Peak serum theophylline levels occurred at 6.9±5.2 (SD) hours, with a normalized (to 800 mg) peak level being 6.2±2.1 (SD). The apparent elimination half-life for the 400 mg Theophylline (Anhydrous) Extended-Release Tablets was 17.2±5.8 (SD) hours.
Steady-state pharmacokinetics were determined in a study in 12 fasted patients with chronic reversible obstructive pulmonary disease. All were dosed with two 400 mg Theophylline (Anhydrous) Extended-Release Tablets given once daily in the morning and a reference controlled-release BID product administered as two 200 mg tablets given 12 hours apart. The pharmacokinetic parameters obtained for Theophylline (Anhydrous) Extended-Release Tablets given at doses of 800 mg once daily in the morning were virtually identical to the corresponding parameters for the reference drug when given as 400 mg BID. In particular, the AUC, Cmax and Cmin values obtained in this study were as follows:
Theophylline (Anhydrous)
Extended-Release Tablets
800 mg
Q24h±S.D.Reference Drug
400 mg
Q12h±S.D.AUC, (0-24 hours), mcg hr/mL 288.9±21.5 283.5±38.4 Cmax, mcg/mL 15.7±2.8 15.2±2.1 Cmin, mcg/mL 7.9±1.6 7.8±1.7 Cmax-Cmin diff. 7.7±1.5 7.4±1.5 Single-dose studies in which subjects were fasted for twelve (12) hours prior to and an additional four (4) hours following dosing, demonstrated reduced bioavailability as compared to dosing with food. One single-dose study in 20 normal volunteers dosed with two (2) 400 mg tablets in the morning, compared dosing under these fasting conditions with dosing immediately prior to a standardized breakfast (769 calories, consisting of 97 grams carbohydrates, 33 grams protein and 27 grams fat). Under fed conditions, the pharmacokinetic parameters were: AUC=231.7±92.4 mcg hr/mL, Cmax=8.4±2.6 mcg/mL, Tmax=17.3±6.7 hours. Under fasting conditions, these parameters were AUC=141.2±6.53 mcg hr/mL, Cmax=5.5±1.5 mcg/mL, Tmax=6.5±2.1 hours.
Another single-dose study in 21 normal male volunteers, dosed in the evening, compared fasting to a standardized high calorie, high fat meal (870-1,020 calories, consisting of 33 grams protein, 55-75 grams fat, 58 grams carbohydrates). In the fasting arm subjects received one Theophylline (Anhydrous) Extended-Release 400 mg Tablet at 8 p.m. after an eight hour fast followed by a further four hour fast. In the fed arm, subjects were again dosed with one 400 mg Theophylline (Anhydrous) Extended-Release Tablet, but at 8 p.m. immediately after the high fat content standardized meal cited above. The pharmacokinetic parameters (normalized to 800 mg) fed were AUC=221.8±40.9 mcg hr/mL, Cmax=10.9±1.7 mcg/mL, Tmax=11.8±2.2 hours. In the fasting arm, the pharmacokinetic parameters (normalized to 800 mg) were AUC=146.4±40.9 mcg hr/mL, Cmax= 6.7±1.7 mcg/mL, Tmax=7.3±2.2 hours.
Thus, administration of single Theophylline (Anhydrous) Extended-Release doses to healthy normal volunteers, under prolonged fasted conditions (at least 10 hour overnight fast before dosing followed by an additional four (4) hour fast after dosing) results in decreased bioavailability. However, there was no failure of this delivery system leading to a sudden and unexpected release of a large quantity of theophylline with Theophylline (Anhydrous) Extended-Release Tablets even when they are administered with a high fat, high calorie meal.
Similar studies were conducted with the 600 mg Theophylline (Anhydrous) Extended-Release Tablet. A single-dose study in 24 subjects with an established theophylline clearance of ≤ 4 L/hr, compared the pharmacokinetic evaluation of one 600 mg Theophylline (Anhydrous) Extended-Release Tablet and one and one-half 400 mg Theophylline (Anhydrous) Extended-Release Tablets under fed (using a standard high fat diet) and fasted conditions. The results of this 4-way randomized crossover study demonstrate the bioequivalence of the 400 mg and 600 mg Theophylline (Anhydrous) Extended-Release Tablets. Under fed conditions, the pharmacokinetic results for the one and one-half 400 mg tablets were AUC=214.64±55.88 mcg hr/mL, Cmax=10.58±2.21 mcg/mL and Tmax=9.00±2.64 hours, and for the 600 mg tablet were AUC=207.85±48.9 mcg hr/mL, Cmax=10.39±1.91 mcg/mL and Tmax=9.58±1.86 hours. Under fasted conditions the pharmacokinetic results for the one and one-half 400 mg tablets were AUC=191.85±51.1 mcg hr/mL, Cmax=7.37±1.83 mcg/mL and Tmax=8.08±4.39 hours; and for the 600 mg tablet were AUC=199.39±70.27 mcg hr/mL, Cmax=7.66±2.09 mcg/mL and Tmax=9.67±4.89 hours.
In this study the mean fed/fasted ratios for the one and one-half 400 mg tablets and the 600 mg tablet were about 112% and 104%, respectively.
In another study, the bioavailability of the 600 mg Theophylline (Anhydrous) Extended-Release Tablet was examined with morning and evening administration. This single-dose, crossover study in 22 healthy males was conducted under fed (standard high fat diet) conditions. The results demonstrated no clinically significant difference in the bioavailability of the 600 mg Theophylline (Anhydrous) Extended-Release Tablet administered in the morning or in the evening. The results were: AUC=233.6±45.1 mcg hr/mL, Cmax=10.6±1.3 mcg/mL and Tmax=12.5±3.2 hours with morning dosing; AUC=209.8±46.2 mcg hr/mL, Cmax=9.7±1.4 mcg/mL and Tmax=13.7± 3.3 hours with evening dosing. The PM/AM ratio was 89.3%.
The absorption characteristics of Theophylline (Anhydrous) Extended-Release Tablets (theophylline, anhydrous) have been extensively studied. A steady-state crossover bioavailability study in 22 normal males compared two Theophylline (Anhydrous) Extended-Release 400 mg Tablets administered q24h at 8 a.m. immediately after breakfast with a reference controlled-release theophylline product administered BID in fed subjects at 8 a.m. immediately after breakfast and 8 p.m. immediately after dinner (769 calories, consisting of 97 grams carbohydrates, 33 grams protein and 27 grams fat). The pharmacokinetic parameters for Theophylline (Anhydrous) Extended-Release 400 mg Tablets under these steady-state conditions were AUC=203.3±87.1 mcg hr/mL, Cmax=12.1±3.8 mcg/mL, Cmin=4.50±3.6, Tmax=8.8±4.6 hours. For the reference BID product, the pharmacokinetic parameters were AUC=219.2±88.4 mcg hr/mL, Cmax=11.0±4.1 mcg/mL, Cmin=7.28±3.5, Tmax=6.9±3.4 hours. The mean percent fluctuation [(Cmax-Cmin/Cmin)×100]=169% for the once-daily regimen and 51% for the reference product BID regimen.
The bioavailability of the 600 mg Theophylline (Anhydrous) Extended-Release Tablet was further evaluated in a multiple dose, steady-state study in 26 healthy males comparing the 600 mg Tablet to one and one-half 400 mg Theophylline (Anhydrous) Extended-Release Tablets. All subjects had previously established theophylline clearances of ≤4 L/hr and were dosed once‑daily for 6 days under fed conditions. The results showed no clinically significant difference between the 600 mg and one and one-half 400 mg Theophylline (Anhydrous) Extended-Release Tablet regimens. Steady-state results were:
600 MG TABLET
FED600 MG
(ONE + ONE-HALF 400 MG TABLETS)
FEDAUC 0-24 hrs (mcg hr/mL) 209.77±51.04 212.32±56.29 Cmax (mcg/mL) 12.91±2.46 13.17±3.11 Cmin (mcg/mL) 5.52±1.79 5.39±1.95 Tmax (hours) 8.62±3.21 7.23±2.35 Percent Fluctuation 183.73±54.02 179.72±28.86 The bioavailability ratio for the 600/400 mg tablets was 98.8%. Thus, under all study conditions the 600 mg tablet is bioequivalent to one and one-half 400 mg tablets.
Studies demonstrate that as long as subjects were either consistently fed or consistently fasted, there is similar bioavailability with once-daily administration of Theophylline (Anhydrous) Extended-Release Tablets whether dosed in the morning or evening.
Distribution
Once theophylline enters the systemic circulation, about 40% is bound to plasma protein, primarily albumin. Unbound theophylline distributes throughout body water, but distributes poorly into body fat. The apparent volume of distribution of theophylline is approximately 0.45 L/kg (range 0.3-0.7 L/kg) based on ideal body weight. Theophylline passes freely across the placenta, into breast milk and into the cerebrospinal fluid (CSF). Saliva theophylline concentrations approximate unbound serum concentrations, but are not reliable for routine or therapeutic monitoring unless special techniques are used. An increase in the volume of distribution of theophylline, primarily due to reduction in plasma protein binding, occurs in premature neonates, patients with hepatic cirrhosis, uncorrected acidemia, the elderly and in women during the third trimester of pregnancy. In such cases, the patient may show signs of toxicity at total (bound+unbound) serum concentrations of theophylline in the therapeutic range (10-20 mcg/mL) due to elevated concentrations of the pharmacologically active unbound drug. Similarly, a patient with decreased theophylline binding may have a sub-therapeutic total drug concentration while the pharmacologically active unbound concentration is in the therapeutic range. If only total serum theophylline concentration is measured, this may lead to an unnecessary and potentially dangerous dose increase. In patients with reduced protein binding, measurement of unbound serum theophylline concentration provides a more reliable means of dosage adjustment than measurement of total serum theophylline concentration. Generally, concentrations of unbound theophylline should be maintained in the range of 6-12 mcg/mL.
Metabolism
Following oral dosing, theophylline does not undergo any measurable first-pass elimination. In adults and children beyond one year of age, approximately 90% of the dose is metabolized in the liver. Biotransformation takes place through demethylation to 1-methylxanthine and 3-methylxanthine and hydroxylation to 1,3-dimethyluric acid. 1-methylxanthine is further hydroxylated, by xanthine oxidase, to 1-methyluric acid. About 6% of a theophylline dose is N-methylated to caffeine. Theophylline demethylation to 3-methylxanthine is catalyzed by cytochrome P-450 1A2, while cytochromes P-450 2E1 and P-450 3A3 catalyze the hydroxylation to 1,3-dimethyluric acid. Demethylation to 1-methylxanthine appears to be catalyzed either by cytochrome P-450 1A2 or a closely related cytochrome. In neonates, the N-demethylation pathway is absent while the function of the hydroxylation pathway is markedly deficient. The activity of these pathways slowly increases to maximal levels by one year of age.
Caffeine and 3-methylxanthine are the only theophylline metabolites with pharmacologic activity. 3-methylxanthine has approximately one tenth the pharmacologic activity of theophylline and serum concentrations in adults with normal renal function are <1 mcg/mL. In patients with end-stage renal disease, 3-methylxanthine may accumulate to concentrations that approximate the unmetabolized theophylline concentration. Caffeine concentrations are usually undetectable in adults regardless of renal function. In neonates, caffeine may accumulate to concentrations that approximate the unmetabolized theophylline concentration and thus, exert a pharmacologic effect.
Both the N-demethylation and hydroxylation pathways of theophylline biotransformation are capacity-limited. Due to the wide intersubject variability of the rate of theophylline metabolism, non-linearity of elimination may begin in some patients at serum theophylline concentrations <10 mcg/mL. Since this non-linearity results in more than proportional changes in serum theophylline concentrations with changes in dose, it is advisable to make increases or decreases in dose in small increments in order to achieve desired changes in serum theophylline concentrations (see DOSAGE AND ADMINISTRATION, Table VI). Accurate prediction of dose-dependency of theophylline metabolism in patients a priori is not possible, but patients with very high initial clearance rates (i.e., low steady-state serum theophylline concentrations at above average doses) have the greatest likelihood of experiencing large changes in serum theophylline concentration in response to dosage changes.
Excretion
In neonates, approximately 50% of the theophylline dose is excreted unchanged in the urine. Beyond the first three months of life, approximately 10% of the theophylline dose is excreted unchanged in the urine. The remainder is excreted in the urine mainly as 1,3-dimethyluric acid (35-40%), 1-methyluric acid (20-25%) and 3-methylxanthine (15-20%). Since little theophylline is excreted unchanged in the urine and since active metabolites of theophylline (i.e., caffeine, 3-methylxanthine) do not accumulate to clinically significant levels even in the face of end-stage renal disease, no dosage adjustment for renal insufficiency is necessary in adults and children >3 months of age. In contrast, the large fraction of the theophylline dose excreted in the urine as unchanged theophylline and caffeine in neonates requires careful attention to dose reduction and frequent monitoring of serum theophylline concentrations in neonates with reduced renal function (See WARNINGS).
Serum Concentrations at Steady-State
After multiple doses of theophylline, steady-state is reached in 30-65 hours (average 40 hours) in adults. At steady-state, on a dosage regimen with 24-hour intervals, the expected mean trough concentration is approximately 50% of the mean peak concentration, assuming a mean theophylline half-life of 8 hours. The difference between peak and trough concentrations is larger in patients with more rapid theophylline clearance. In these patients administration of Theophylline (Anhydrous) Extended-Release Tablets may be required more frequently (every 12 hours).
Special Populations
(See Table I for mean clearance and half-life values)
Geriatric
The clearance of theophylline is decreased by an average of 30% in healthy elderly adults (>60 yrs) compared to healthy young adults. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in elderly patients (see WARNINGS).
Pediatrics
The clearance of theophylline is very low in neonates (see WARNINGS). Theophylline clearance reaches maximal values by one year of age, remains relatively constant until about 9 years of age and then slowly decreases by approximately 50% to adult values at about age 16. Renal excretion of unchanged theophylline in neonates amounts to about 50% of the dose, compared to about 10% in children older than three months and in adults. Careful attention to dosage selection and monitoring of serum theophylline concentrations are required in pediatric patients (see WARNINGS and DOSAGE AND ADMINISTRATION).
Gender
Gender differences in theophylline clearance are relatively small and unlikely to be of clinical significance. Significant reduction in theophylline clearance, however, has been reported in women on the 20th day of the menstrual cycle and during the third trimester of pregnancy.
Renal Insufficiency
Only a small fraction, e.g., about 10%, of the administered theophylline dose is excreted unchanged in the urine of children greater than three months of age and adults. Since little theophylline is excreted unchanged in the urine and since active metabolites of theophylline (i.e., caffeine, 3-methylxanthine) do not accumulate to clinically significant levels even in the face of end-stage renal disease, no dosage adjustment for renal insufficiency is necessary in adults and children >3 months of age. In contrast, approximately 50% of the administered theophylline dose is excreted unchanged in the urine in neonates. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in neonates with decreased renal function (see WARNINGS).
Hepatic Insufficiency
Theophylline clearance is decreased by 50% or more in patients with hepatic insufficiency (e.g., cirrhosis, acute hepatitis, cholestasis). Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with reduced hepatic function (see WARNINGS).
Congestive Heart Failure (CHF)
Theophylline clearance is decreased by 50% or more in patients with CHF. The extent of reduction in theophylline clearance in patients with CHF appears to be directly correlated to the severity of the cardiac disease. Since theophylline clearance is independent of liver blood flow, the reduction in clearance appears to be due to impaired hepatocyte function rather than reduced perfusion. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with CHF (see WARNINGS).
Smokers
Tobacco and marijuana smoking appears to increase the clearance of theophylline by induction of metabolic pathways. Theophylline clearance has been shown to increase by approximately 50% in young adult tobacco smokers and by approximately 80% in elderly tobacco smokers compared to non-smoking subjects. Passive smoke exposure has also been shown to increase theophylline clearance by up to 50%. Abstinence from tobacco smoking for one week causes a reduction of approximately 40% in theophylline clearance. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients who stop smoking (see WARNINGS). Use of nicotine gum has been shown to have no effect on theophylline clearance.
Fever
Fever, regardless of its underlying cause, can decrease the clearance of theophylline. The magnitude and duration of the fever appear to be directly correlated to the degree of decrease of theophylline clearance. Precise data are lacking, but a temperature of 39°C (102°F) for at least 24 hours is probably required to produce a clinically significant increase in serum theophylline concentrations. Children with rapid rates of theophylline clearance (i.e., those who require a dose that is substantially larger than average [e.g., >22 mg/kg/day] to achieve a therapeutic peak serum theophylline concentration when afebrile) may be at greater risk of toxic effects from decreased clearance during sustained fever. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with sustained fever (see WARNINGS).
Miscellaneous
Other factors associated with decreased theophylline clearance include the third trimester of pregnancy, sepsis with multiple organ failure, and hypothyroidism. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with any of these conditions (see WARNINGS). Other factors associated with increased theophylline clearance include hyperthyroidism and cystic fibrosis.
CLINICAL STUDIES:
In patients with chronic asthma, including patients with severe asthma requiring inhaled corticosteroids or alternate-day oral corticosteroids, many clinical studies have shown that theophylline decreases the frequency and severity of symptoms, including nocturnal exacerbations, and decreases the "as needed" use of inhaled beta-2 agonists. Theophylline has also been shown to reduce the need for short courses of daily oral prednisone to relieve exacerbations of airway obstruction that are unresponsive to bronchodilators in asthmatics.
In patients with chronic obstructive pulmonary disease (COPD), clinical studies have shown that theophylline decreases dyspnea, air trapping, the work of breathing, and improves contractility of diaphragmatic muscles with little or no improvement in pulmonary function measurements.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Concurrent Illness
Theophylline should be used with extreme caution in patients with the following clinical conditions due to the increased risk of exacerbation of the concurrent condition:
- Active peptic ulcer disease
- Seizure disorders
- Cardiac arrhythmias (not including bradyarrhythmias)
Conditions That Reduce Theophylline Clearance
There are several readily identifiable causes of reduced theophylline clearance. If the total daily dose is not appropriately reduced in the presence of these risk factors, severe and potentially fatal theophylline toxicity can occur. Careful consideration must be given to the benefits and risks of theophylline use and the need for more intensive monitoring of serum theophylline concentrations in patients with the following risk factors:
Concurrent Diseases
- Acute pulmonary edema
- Congestive heart failure
- Cor-pulmonale
- Fever; ≥102° for 24 hours or more; or lesser temperature elevations for longer periods
- Hypothyroidism
- Liver disease; cirrhosis, acute hepatitis
- Reduced renal function in infants <3 months of age
- Sepsis with multi-organ failure
- Shock
Drug Interactions
Adding a drug that inhibits theophylline metabolism (e.g., cimetidine, erythromycin, tacrine) or stopping a concurrently administered drug that enhances theophylline metabolism (e.g., carbamazepine, rifampin). (see PRECAUTIONS, Drug Interactions, Table II).
When Signs or Symptoms of Theophylline Toxicity Are Present
Whenever a patient receiving theophylline develops nausea or vomiting, particularly repetitive vomiting, or other signs or symptoms consistent with theophylline toxicity (even if another cause may be suspected), additional doses of theophylline should be withheld and a serum theophylline concentration measured immediately. Patients should be instructed not to continue any dosage that causes adverse effects and to withhold subsequent doses until the symptoms have resolved, at which time the healthcare professional may instruct the patient to resume the drug at a lower dosage (see DOSAGE AND ADMINISTRATION, Dosing Guidelines, Table VI).
Dosage Increases
Increases in the dose of theophylline should not be made in response to an acute exacerbation of symptoms of chronic lung disease since theophylline provides little added benefit to inhaled beta2-selective agonists and systemically administered corticosteroids in this circumstance and increases the risk of adverse effects. A peak steady‑state serum theophylline concentration should be measured before increasing the dose in response to persistent chronic symptoms to ascertain whether an increase in dose is safe. Before increasing the theophylline dose on the basis of a low serum concentration, the healthcare professional should consider whether the blood sample was obtained at an appropriate time in relationship to the dose and whether the patient has adhered to the prescribed regimen (see PRECAUTIONS, Laboratory Tests).
As the rate of theophylline clearance may be dose-dependent (i.e., steady-state serum concentrations may increase disproportionately to the increase in dose), an increase in dose based upon a sub-therapeutic serum concentration measurement should be conservative. In general, limiting dose increases to about 25% of the previous total daily dose will reduce the risk of unintended excessive increases in serum theophylline concentration (see DOSAGE AND ADMINISTRATION, Table VI).
-
PRECAUTIONS
General
Careful consideration of the various interacting drugs and physiologic conditions that can alter theophylline clearance and require dosage adjustment should occur prior to initiation of theophylline therapy, prior to increases in theophylline dose, and during follow up (see WARNINGS). The dose of theophylline selected for initiation of therapy should be low and, if tolerated, increased slowly over a period of a week or longer with the final dose guided by monitoring serum theophylline concentrations and the patient's clinical response (see DOSAGE AND ADMINISTRATION, Table V). Monitoring Serum Theophylline Concentrations: Serum theophylline concentration measurements are readily available and should be used to determine whether the dosage is appropriate. Specifically, the serum theophylline concentration should be measured as follows:
- When initiating therapy to guide final dosage adjustment after titration.
- Before making a dose increase to determine whether the serum concentration is sub-therapeutic in a patient who continues to be symptomatic.
- Whenever signs or symptoms of theophylline toxicity are present.
- Whenever there is a new illness, worsening of a chronic illness or a change in the patient's treatment regimen that may alter theophylline clearance (e.g., fever >102°F sustained for ≥24 hours, hepatitis, or drugs listed in Table II are added or discontinued).
To guide a dose increase, the blood sample should be obtained at the time of the expected peak serum theophylline concentration; 12 hours after an evening dose or 9 hours after a morning dose at steady-state. For most patients, steady‑state will be reached after 3 days of dosing when no doses have been missed, no extra doses have been added, and none of the doses have been taken at unequal intervals. A trough concentration (i.e., at the end of the dosing interval) provides no additional useful information and may lead to an inappropriate dose increase since the peak serum theophylline concentration can be two or more times greater than the trough concentration with an immediate-release formulation. If the serum sample is drawn more than 12 hours after the evening dose, or more than 9 hours after a morning dose, the results must be interpreted with caution since the concentration may not be reflective of the peak concentration. In contrast, when signs or symptoms of theophylline toxicity are present, a serum sample should be obtained as soon as possible, analyzed immediately, and the result reported to the healthcare professional without delay. In patients in whom decreased serum protein binding is suspected (e.g., cirrhosis, women during the third trimester of pregnancy), the concentration of unbound theophylline should be measured and the dosage adjusted to achieve an unbound concentration of 6-12 mcg/mL.
Saliva concentrations of theophylline cannot be used reliably to adjust dosage without special techniques.
Effects on Laboratory Tests
As a result of its pharmacological effects, theophylline at serum concentrations within the 10-20 mcg/mL range modestly increases plasma glucose (from a mean of 88 mg% to 98 mg%), uric acid (from a mean of 4 mg/dL to 6 mg/dL), free fatty acids (from a mean of 451 µEq/L to 800 µEq/L), total cholesterol (from a mean of 140 vs 160 mg/dL), HDL (from a mean of 36 to 50 mg/dL), HDL/LDL ratio (from a mean of 0.5 to 0.7), and urinary free cortisol excretion (from a mean of 44 to 63 mcg/24 hr). Theophylline at serum concentrations within the 10-20 mcg/mL range may also transiently decrease serum concentrations of triiodothyronine (144 before, 131 after one week and 142 ng/dL after 4 weeks of theophylline). The clinical importance of these changes should be weighed against the potential therapeutic benefit of theophylline in individual patients.
Information for Patients
The patient (or parent/care giver) should be instructed to seek medical advice whenever nausea, vomiting, persistent headache, insomnia or rapid heartbeat occurs during treatment with theophylline, even if another cause is suspected. The patient should be instructed to contact their healthcare professional if they develop a new illness, especially if accompanied by a persistent fever, if they experience worsening of a chronic illness, if they start or stop smoking cigarettes or marijuana, or if another healthcare professional adds a new medication or discontinues a previously prescribed medication. Patients should be informed that theophylline interacts with a wide variety of drugs (see Table II). The dietary supplement St. John's Wort (Hypericum perforatum) should not be taken at the same time as theophylline, since it may result in decreased theophylline levels. If patients are already taking St. John's Wort and theophylline together, they should consult their healthcare professional before stopping the St. John's Wort, since their theophylline concentrations may rise when this is done, resulting in toxicity. Patients should be instructed to inform all healthcare professional involved in their care that they are taking theophylline, especially when a medication is being added or deleted from their treatment. Patients should be instructed to not alter the dose, timing of the dose, or frequency of administration without first consulting their healthcare professional. If a dose is missed, the patient should be instructed to take the next dose at the usually scheduled time and to not attempt to make up for the missed dose.
Theophylline (Anhydrous) Extended-Release Tablets can be taken once a day in the morning or evening. It is recommended that Theophylline (Anhydrous) Extended-Release Tablets be taken with meals. Patients should be advised that if they choose to take Theophylline (Anhydrous) Extended-Release Tablets with food it should be taken consistently with food and if they take it in a fasted condition it should routinely be taken fasted. It is important that the product whenever dosed be dosed consistently with or without food.
Theophylline (Anhydrous) Extended-Release Tablets are not to be chewed or crushed because it may lead to a rapid release of theophylline with the potential for toxicity. The scored tablet may be split. Patients receiving Theophylline (Anhydrous) Extended-Release Tablets may pass an intact matrix tablet in the stool or via colostomy. These matrix tablets usually contain little or no residual theophylline.
Drug Interactions
Theophylline interacts with a wide variety of drugs. The interaction may be pharmacodynamic, i.e., alterations in the therapeutic response to theophylline or another drug or occurrence of adverse effects without a change in serum theophylline concentration. More frequently, however, the interaction is pharmacokinetic, i.e., the rate of theophylline clearance is altered by another drug resulting in increased or decreased serum theophylline concentrations. Theophylline only rarely alters the pharmacokinetics of other drugs.
The drugs listed in Table II have the potential to produce clinically significant pharmacodynamic or pharmacokinetic interactions with theophylline. The information in the "Effect" column of Table II assumes that the interacting drug is being added to a steady-state theophylline regimen. If theophylline is being initiated in a patient who is already taking a drug that inhibits theophylline clearance (e.g., cimetidine, erythromycin), the dose of theophylline required to achieve a therapeutic serum theophylline concentration will be smaller. Conversely, if theophylline is being initiated in a patient who is already taking a drug that enhances theophylline clearance (e.g., rifampin), the dose of theophylline required to achieve a therapeutic serum theophylline concentration will be larger. Discontinuation of a concomitant drug that increases theophylline clearance will result in accumulation of theophylline to potentially toxic levels, unless the theophylline dose is appropriately reduced. Discontinuation of a concomitant drug that inhibits theophylline clearance will result in decreased serum theophylline concentrations, unless the theophylline dose is appropriately increased.
The drugs listed in Table III have either been documented not to interact with theophylline or do not produce a clinically significant interaction (i.e., <15% change in theophylline clearance).
The listing of drugs in Table II and III are current as of February 9, 1995. New interactions are continuously being reported for theophylline, especially with new chemical entities. The healthcare professional should not assume that a drug does not interact with theophylline if it is not listed in Table II. Before addition of a newly available drug in a patient receiving theophylline, the package insert of the new drug and/or the medical literature should be consulted to determine if an interaction between the new drug and theophylline has been reported.
Table II. Clinically significant drug interactions with theophylline*. Drug Type of Interaction Effect† - * Refer to PRECAUTIONS, Drug Interactions for further information regarding table.
- † Average effect on steady-state theophylline concentration or other clinical effect for pharmacologic interactions. Individual patients may experience larger changes in serum theophylline concentration than the value listed.
Adenosine Theophylline blocks adenosine receptors. Higher doses of adenosine may be required to achieve desired effect. Alcohol A single large dose of alcohol (3 mL/kg of whiskey) decreases theophylline clearance for up to 24 hours 30% increase Allopurinol Decreases theophylline clearance at allopurinol doses ≥600 mg/day. 25% increase Aminoglutethimide Increases theophylline clearance by induction of microsomal enzyme activity 25% decrease Carbamazepine Similar to aminoglutethimide. 30% decrease Cimetidine Decreases theophylline clearance by inhibiting cytochrome P450 1A2. 70% increase Ciprofloxacin Similar to cimetidine. 40% increase Clarithromycin Similar to erythromycin. 25% increase Diazepam Benzodiazepines increase CNS concentrations of adenosine, a potent CNS depressant, while theophylline blocks adenosine receptors. Larger diazepam doses may be required to produce desired level of sedation. Discontinuation of theophylline without reduction of diazepam dose may result in respiratory depression. Disulfiram Decreases theophylline clearance by inhibiting hydroxylation and demethylation. 50% increase Enoxacin Similar to cimetidine. 300% increase Ephedrine Synergistic CNS effects Increased frequency of nausea, nervousness, and insomnia. Erythromycin Erythromycin metabolite decreases theophylline clearance by inhibiting cytochrome P450 3A3. 35% increase. Erythromycin steady-state serum concentrations decrease by a similar amount. Estrogen Estrogen containing oral contraceptives decrease theophylline clearance in a dose-dependent fashion. The effect of progesterone on theophylline clearance is unknown. 30% increase Flurazepam Similar to diazepam. Similar to diazepam. Fluvoxamine Similar to cimetidine. Similar to cimetidine. Halothane Halothane sensitizes the myocardium to catecholamines, theophylline increases release of endogenous catecholamines. Increased risk of ventricular arrhythmias. Interferon, human recombinant alpha-A Decreases theophylline clearance. 100% increase Isoproterenol (IV) Increases theophylline clearance. 20% decrease Ketamine Pharmacologic May lower theophylline seizure threshold. Lithium Theophylline increases renal lithium clearance. Lithium dose required to achieve a therapeutic serum concentration increased an average of 60%. Lorazepam Similar to diazepam. Similar to diazepam. Methotrexate (MTX) Decreases theophylline clearance. 20% increase after low dose MTX, higher dose MTX may have a greater effect. Mexiletine Similar to disulfiram. 80% increase Midazolam Similar to diazepam. Similar to diazepam. Moricizine Increases theophylline clearance. 25% decrease Pancuronium Theophylline may antagonize non-depolarizing neuromuscular blocking effects; possibly due to phosphodiesterase inhibition. Larger dose of pancuronium may be required to achieve neuromuscular blockade. Pentoxifylline Decreases theophylline clearance. 30% increase Phenobarbital (PB) Similar to aminoglutethimide. 25% decrease after two weeks of concurrent PB. Phenytoin Phenytoin increases theophylline clearance by increasing microsomal enzyme activity. Theophylline decreases phenytoin absorption. Serum theophylline and phenytoin concentrations decrease about 40%. Propafenone Decreases theophylline clearance and pharmacologic interaction. 40% increase. Beta-2 blocking effect may decrease efficacy of theophylline. Propranolol Similar to cimetidine and pharmacologic interaction. 100% increase. Beta-2 blocking effect may decrease efficacy of theophylline. Rifampin Increases theophylline clearance by increasing cytochrome P450 1A2 and 3A3 activity. 20-40% decrease St. John's Wort (Hypericum Perforatum) Decrease in theophylline plasma concentrations. Higher doses of theophylline may be required to achieve desired effect. Stopping St. John's Wort may result in theophylline toxicity. Sulfinpyrazone Increases theophylline clearance by increasing demethylation and hydroxylation. Decreases renal clearance of theophylline. 20% decrease Tacrine Similar to cimetidine, also increases renal clearance of theophylline. 90% increase Thiabendazole Decreases theophylline clearance. 190% increase Ticlopidine Decreases theophylline clearance. 60% increase Troleandomycin Similar to erythromycin. 33-100% increase depending on troleandomycin dose. Verapamil Similar to disulfiram. 20% increase Table III. Drugs that have been documented not to interact with theophylline or drugs that produce no clinically significant interaction with theophylline.* - * Refer to PRECAUTIONS, Drug Interactions for information regarding table.
albuterol, systemic and inhaled mebendazole amoxicillin medroxyprogesterone ampicillin, with or without sulbactam methylprednisolone atenolol metronidazole azithromycin metoprolol caffeine, dietary ingestion nadolol cefaclor nifedipine co-trimoxazole (trimethoprim and sulfamethoxazole) nizatidine diltiazem norfloxacin dirithromycin ofloxacin enflurane omeprazole famotidine prednisone, prednisolone felodipine ranitidine finasteride rifabutin hydrocortisone roxithromycin isoflurane Sorbitol (purgative doses do not inhibit theophylline absorption) isoniazid sucralfate isradipine terbutaline, systemic influenza vaccine terfenadine ketoconazole tetracycline lomefloxacin tocainide Drug-Food Interactions
The bioavailability of Theophylline (Anhydrous) Extended-Release Tablets has been studied with co‑administration of food. In three single-dose studies, subjects given Theophylline (Anhydrous) Extended-Release 400 mg or 600 mg Tablets with a standardized high-fat meal were compared to fasted conditions. Under fed conditions, the peak plasma concentration and bioavailability were increased; however, a precipitous increase in the rate and extent of absorption was not evident (See Pharmacokinetics, Absorption). The increased peak and extent of absorption under fed conditions suggests that dosing should be ideally administered consistently either with or without food.
The Effect of Other Drugs on Theophylline Serum Concentration Measurements
Most serum theophylline assays in clinical use are immunoassays which are specific for theophylline. Other xanthines such as caffeine, dyphylline, and pentoxifylline are not detected by these assays. Some drugs (e.g., cefazolin, cephalothin), however, may interfere with certain HPLC techniques. Caffeine and xanthine metabolites in neonates or patients with renal dysfunction may cause the reading from some dry reagent office methods to be higher than the actual serum theophylline concentration.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long term carcinogenicity studies have been carried out in mice (oral doses 30-150 mg/kg) and rats (oral doses 5-75 mg/kg). Results are pending.
Theophylline has been studied in Ames salmonella, in vivo and in vitro cytogenetics, micronucleus and Chinese hamster ovary test systems and has not been shown to be genotoxic.
In a 14 week continuous breeding study, theophylline, administered to mating pairs of B6C3F1 mice at oral doses of 120, 270 and 500 mg/kg (approximately 1.0-3.0 times the human dose on a mg/m2 basis) impaired fertility, as evidenced by decreases in the number of live pups per litter, decreases in the mean number of litters per fertile pair, and increases in the gestation period at the high dose as well as decreases in the proportion of pups born alive at the mid and high dose. In 13 week toxicity studies, theophylline was administered to F344 rats and B6C3F1 mice at oral doses of 40-300 mg/kg (approximately 2.0 times the human dose on a mg/m2 basis). At the high dose, systemic toxicity was observed in both species including decreases in testicular weight.
Teratogenic Effects
Category C
In studies in which pregnant mice, rats and rabbits were dosed during the period of organogenesis, theophylline produced teratogenic effects.
In studies with mice, a single intraperitoneal dose at and above 100 mg/kg (approximately equal to the maximum recommended oral dose for adults on a mg/m2 basis) during organogenesis produced cleft palate and digital abnormalities. Micromelia, micrognathia, clubfoot, subcutaneous hematoma, open eyelids, and embryolethality were observed at doses that are approximately 2 times the maximum recommended oral dose for adults on a mg/m2 basis.
In a study with rats dosed from conception through organogenesis, an oral dose of 150 mg/kg/day (approximately 2 times the maximum recommended oral dose for adults on a mg/m2 basis) produced digital abnormalities. Embryolethality was observed with a subcutaneous dose of 200 mg/kg/day (approximately 4 times the maximum recommended oral dose for adults on a mg/m2 basis).
In a study in which pregnant rabbits were dosed throughout organogenesis, an intravenous dose of 60 mg/kg/day (approximately 2 times the maximum recommended oral dose for adults on a mg/m2 basis), which caused the death of one doe and clinical signs in others, produced cleft palate and was embryolethal. Doses at and above 15 mg/kg/day (less than the maximum recommended oral dose for adults on a mg/m2 basis) increased the incidence of skeletal variations.
There are no adequate and well-controlled studies in pregnant women. Theophylline should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Theophylline is excreted into breast milk and may cause irritability or other signs of mild toxicity in nursing human infants. The concentration of theophylline in breast milk is about equivalent to the maternal serum concentration. An infant ingesting a liter of breast milk containing 10-20 mcg/mL of theophylline per day is likely to receive 10-20 mg of theophylline per day. Serious adverse effects in the infant are unlikely unless the mother has toxic serum theophylline concentrations.
Pediatric Use
Theophylline is safe and effective for the approved indications in pediatric patients. The maintenance dose of theophylline must be selected with caution in pediatric patients since the rate of theophylline clearance is highly variable across the pediatric age range (see CLINICAL PHARMACOLOGY, Table I, WARNINGS, and DOSAGE AND ADMINISTRATION, Table V).
Geriatric Use
Elderly patients are at significantly greater risk of experiencing serious toxicity from theophylline than younger patients due to pharmacokinetic and pharmacodynamic changes associated with aging. The clearance of theophylline is decreased by an average of 30% in healthy elderly adults (>60 yrs) compared to healthy young adults. Theophylline clearance may be further reduced by concomitant diseases prevalent in the elderly, which further impair clearance of this drug and have the potential to increase serum levels and potential toxicity. These conditions include impaired renal function, chronic obstructive pulmonary disease, congestive heart failure, hepatic disease and an increased prevalence of use of certain medications (see PRECAUTIONS: Drug Interactions) with the potential for pharmacokinetic and pharmacodynamic interaction. Protein binding may be decreased in the elderly resulting in an increased proportion of the total serum theophylline concentration in the pharmacologically active unbound form. Elderly patients also appear to be more sensitive to the toxic effects of theophylline after chronic overdosage than younger patients. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in elderly patients (see PRECAUTIONS, Monitoring Serum Theophylline Concentrations, and DOSAGE AND ADMINISTRATION). The maximum daily dose of theophylline in patients greater than 60 years of age ordinarily should not exceed 400 mg/day unless the patient continues to be symptomatic and the peak steady-state serum theophylline concentration is <10 mcg/mL (see DOSAGE AND ADMINISTRATION). Theophylline doses greater than 400 mg/d should be prescribed with caution in elderly patients. Theophylline should be prescribed with caution in elderly male patients with pre-existing partial outflow obstruction, such as prostatic enlargement, due to the risk of urinary retention.
-
ADVERSE REACTIONS
Adverse reactions associated with theophylline are generally mild when peak serum theophylline concentrations are <20 mcg/mL and mainly consist of transient caffeine-like adverse effects such as nausea, vomiting, headache and insomnia. When peak serum theophylline concentrations exceed 20 mcg/mL, however, theophylline produces a wide range of adverse reactions including persistent vomiting, cardiac arrhythmias, and intractable seizures which can be lethal (see OVERDOSAGE). The transient caffeine-like adverse reactions occur in about 50% of patients when theophylline therapy is initiated at doses higher than recommended initial doses (e.g., >300 mg/day in adults and >12 mg/kg/day in children beyond >1 year of age). During the initiation of theophylline therapy, caffeine-like adverse effects may transiently alter patient behavior, especially in school age children, but this response rarely persists. Initiation of theophylline therapy at a low dose with subsequent slow titration to a predetermined age-related maximum dose will significantly reduce the frequency of these transient adverse effects (see DOSAGE AND ADMINISTRATION, Table V). In a small percentage of patients (<3% of children and <10% of adults) the caffeine-like adverse effects persist during maintenance therapy, even at peak serum theophylline concentrations within the therapeutic range (i.e., 10-20 mcg/mL). Dosage reduction may alleviate the caffeine-like adverse effects in these patients, however, persistent adverse effects should result in a reevaluation of the need for continued theophylline therapy and the potential therapeutic benefit of alternative treatment.
Other adverse reactions that have been reported at serum theophylline concentrations <20 mcg/mL include abdominal pain, agitation, anaphylactic reaction, anaphylactiod reaction, anxiety, cardiac arrhythmias, diarrhea, dizziness, fine skeletal muscle tremors, gastric irritation, gastroesophageal reflux, hyperuricemia, irritability, palpitations, pruritus, rash, sinus tachycardia, restlessness, transient diuresis, urinary retention and urticaria. In patients with hypoxia secondary to COPD, multifocal atrial tachycardia and flutter have been reported at serum theophylline concentrations ≥15 mcg/mL. There have been a few isolated reports of seizures at serum theophylline concentrations <20 mcg/mL in patients with an underlying neurological disease or in elderly patients. The occurrence of seizures in elderly patients with serum theophylline concentrations <20 mcg/mL may be secondary to decreased protein binding resulting in a larger proportion of the total serum theophylline concentration in the pharmacologically active unbound form. The clinical characteristics of the seizures reported in patients with serum theophylline concentrations <20 mcg/mL have generally been milder than seizures associated with excessive serum theophylline concentrations resulting from an overdose (i.e., they have generally been transient, often stopped without anticonvulsant therapy, and did not result in neurological residua).
To report SUSPECTED ADVERSE REACTIONS, contact Westminster Pharmaceuticals, LLC at 1-844-221-7294 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Table IV. Manifestations of theophylline toxicity.* Sign/Symptom
Percentage of patients reported with sign or symptom Acute Overdose
(Large Single Ingestion)Chronic Overdosage
(Multiple Excessive Doses)Study 1
(n=157)Study 2
(n=14)Study 1
(n=92)Study 2
(n=102)- * These data are derived from two studies in patients with serum theophylline concentrations >30 mcg/mL. In the first study (Study #1 - Shanon, Ann Intern Med 1993;119:1161-67), data were prospectively collected from 249 consecutive cases of theophylline toxicity referred to a regional poison center for consultation. In the second study (Study #2 - Sessler, Am J Med 1990;88:567-76), data were retrospectively collected from 116 cases with serum theophylline concentrations >30 mcg/mL among 6000 blood samples obtained for measurement of serum theophylline concentrations in three emergency departments. Differences in the incidence of manifestations of theophylline toxicity between the two studies may reflect sample selection as a result of study design (e.g., in Study #1, 48% of the patients had acute intoxications versus only 10% in Study #2) and different methods of reporting results.
- † NR=Not reported in a comparable manner.
Asymptomatic NR† 0 NR† 6 Gastrointestinal Vomiting 73 93 30 61 Abdominal Pain NR† 21 NR† 12 Diarrhea NR† 0 NR† 14 Hematemesis NR† 0 NR† 2 Metabolic/Other Hypokalemia 85 79 44 43 Hyperglycemia 98 NR† 18 NR† Acid/base disturbance 34 21 9 5 Rhabdomyolysis NR† 7 NR† 0 Cardiovascular Sinus tachycardia 100 86 100 62 Other Supraventricular Tachycardias 2 21 12 14 Ventricular premature beats 3 21 10 19 Atrial fibrillation or flutter 1 NR† 12 NR† Multifocal atrial tachycardia 0 NR† 2 NR† Ventricular arrhythmias hemodynamic instability 7 14 40 0 Hypotension/shock NR† 21 NR† 8 Neurologic Nervousness NR† 64 NR† 21 Tremors 38 29 16 14 Disorientation NR† 7 NR† 11 Seizures 5 14 14 5 Death 3 21 10 4 -
OVERDOSAGE
General
The chronicity and pattern of theophylline overdosage significantly influences clinical manifestations of toxicity, management and outcome. There are two common presentations: (1) acute overdose, i.e., ingestion of a single large excessive dose (>10 mg/kg), as occurs in the context of an attempted suicide or isolated medication error, and (2) chronic overdosage, i.e., ingestion of repeated doses that are excessive for the patient's rate of theophylline clearance. The most common causes of chronic theophylline overdosage include patient or caregiver error in dosing, healthcare professional prescribing of an excessive dose or a normal dose in the presence of factors known to decrease the rate of theophylline clearance, and increasing the dose in response to an exacerbation of symptoms without first measuring the serum theophylline concentration to determine whether a dose increase is safe.
Severe toxicity from theophylline overdose is a relatively rare event. In one health maintenance organization, the frequency of hospital admissions for chronic overdosage of theophylline was about 1 per 1000 person-years exposure. In another study, among 6000 blood samples obtained for measurement of serum theophylline concentration, for any reason, from patients treated in an emergency department, 7% were in the 20-30 mcg/mL range and 3% were >30 mcg/mL. Approximately two-thirds of the patients with serum theophylline concentrations in the 20-30 mcg/mL range had one or more manifestations of toxicity while >90% of patients with serum theophylline concentrations >30 mcg/mL were clinically intoxicated. Similarly, in other reports, serious toxicity from theophylline is seen principally at serum concentrations >30 mcg/mL.
Several studies have described the clinical manifestations of theophylline overdose and attempted to determine the factors that predict life-threatening toxicity. In general, patients who experience an acute overdose are less likely to experience seizures than patients who have experienced a chronic overdosage, unless the peak serum theophylline concentration is >100 mcg/mL. After a chronic overdosage, generalized seizures, life-threatening cardiac arrhythmias, and death may occur at serum theophylline concentrations >30 mcg/mL. The severity of toxicity after chronic overdosage is more strongly correlated with the patient's age than the peak serum theophylline concentration; patients >60 years are at the greatest risk for severe toxicity and mortality after a chronic overdosage. Pre-existing or concurrent disease may also significantly increase the susceptibility of a patient to a particular toxic manifestation, e.g., patients with neurologic disorders have an increased risk of seizures and patients with cardiac disease have an increased risk of cardiac arrhythmias for a given serum theophylline concentration compared to patients without the underlying disease.
The frequency of various reported manifestations of theophylline overdose according to the mode of overdose are listed in Table IV.
Other manifestations of theophylline toxicity include increases in serum calcium, creatine kinase, myoglobin and leukocyte count, decreases in serum phosphate and magnesium, acute myocardial infarction, and urinary retention in men with obstructive uropathy.
Seizures associated with serum theophylline concentrations >30 mcg/mL are often resistant to anticonvulsant therapy and may result in irreversible brain injury if not rapidly controlled. Death from theophylline toxicity is most often secondary to cardiorespiratory arrest and/or hypoxic encephalopathy following prolonged generalized seizures or intractable cardiac arrhythmias causing hemodynamic compromise.
Overdose Management
General Recommendations for Patients with Symptoms of Theophylline Overdose or Serum Theophylline Concentrations >30 mcg/mL (Note: Serum theophylline concentrations may continue to increase after presentation of the patient for medical care).
- While simultaneously instituting treatment, contact a regional poison center to obtain updated information and advice on individualizing the recommendations that follow.
- Institute supportive care, including establishment of intravenous access, maintenance of the airway, and electrocardiographic monitoring.
- Treatment of seizures Because of the high morbidity and mortality associated with theophylline-induced seizures, treatment should be rapid and aggressive. Anticonvulsant therapy should be initiated with an intravenous benzodiazepine, e.g., diazepam, in increments of 0.1-0.2 mg/kg every 1-3 minutes until seizures are terminated. Repetitive seizures should be treated with a loading dose of phenobarbital (20 mg/kg infused over 30-60 minutes). Case reports of theophylline overdose in humans and animal studies suggest that phenytoin is ineffective in terminating theophylline-induced seizures. The doses of benzodiazepines and phenobarbital required to terminate theophylline-induced seizures are close to the doses that may cause severe respiratory depression or respiratory arrest; the healthcare professional should therefore be prepared to provide assisted ventilation. Elderly patients and patients with COPD may be more susceptible to the respiratory depressant effects of anticonvulsants. Barbiturate-induced coma or administration of general anesthesia may be required to terminate repetitive seizures or status epilepticus. General anesthesia should be used with caution in patients with theophylline overdose because fluorinated volatile anesthetics may sensitize the myocardium to endogenous catecholamines released by theophylline. Enflurane appears less likely to be associated with this effect than halothane and may, therefore, be safer. Neuromuscular blocking agents alone should not be used to terminate seizures since they abolish the musculoskeletal manifestations without terminating seizure activity in the brain.
- Anticipate Need for Anticonvulsants In patients with theophylline overdose who are at high risk for theophylline-induced seizures, e.g., patients with acute overdoses and serum theophylline concentrations >100 mcg/mL or chronic overdosage in patients >60 years of age with serum theophylline concentrations >30 mcg/mL, the need for anticonvulsant therapy should be anticipated. A benzodiazepine such as diazepam should be drawn into a syringe and kept at the patient's bedside and medical personnel qualified to treat seizures should be immediately available. In selected patients at high risk for theophylline-induced seizures, consideration should be given to the administration of prophylactic anticonvulsant therapy. Situations where prophylactic anticonvulsant therapy should be considered in high risk patients include anticipated delays in instituting methods for extracorporeal removal of theophylline (e.g., transfer of a high risk patient from one healthcare facility to another for extracorporeal removal) and clinical circumstances that significantly interfere with efforts to enhance theophylline clearance (e.g., a neonate where dialysis may not be technically feasible or a patient with vomiting unresponsive to antiemetics who is unable to tolerate multiple-dose oral activated charcoal). In animal studies, prophylactic administration of phenobarbital, but not phenytoin, has been shown to delay the onset of theophylline‑induced generalized seizures and to increase the dose of theophylline required to induce seizures (i.e., markedly increases the LD50). Although there are no controlled studies in humans, a loading dose of intravenous phenobarbital (20 mg/kg infused over 60 minutes) may delay or prevent life-threatening seizures in high risk patients while efforts to enhance theophylline clearance are continued. Phenobarbital may cause respiratory depression, particularly in elderly patients and patients with COPD.
- Treatment of cardiac arrhythmias Sinus tachycardia and simple ventricular premature beats are not harbingers of life‑threatening arrhythmias, they do not require treatment in the absence of hemodynamic compromise, and they resolve with declining serum theophylline concentrations. Other arrhythmias, especially those associated with hemodynamic compromise, should be treated with antiarrhythmic therapy appropriate for the type of arrhythmia.
- Gastrointestinal decontamination Oral activated charcoal (0.5 g/kg up to 20 g and repeat at least once 1-2 hours after the first dose) is extremely effective in blocking the absorption of theophylline throughout the gastrointestinal tract, even when administered several hours after ingestion. If the patient is vomiting, the charcoal should be administered through a nasogastric tube or after administration of an antiemetic. Phenothiazine antiemetics such as prochlorperazine or perphenazine should be avoided since they can lower the seizure threshold and frequently cause dystonic reactions. A single dose of sorbitol may be used to promote stooling to facilitate removal of theophylline bound to charcoal from the gastrointestinal tract. Sorbitol, however, should be dosed with caution since it is a potent purgative which can cause profound fluid and electrolyte abnormalities, particularly after multiple doses. Commercially available fixed combinations of liquid charcoal and sorbitol should be avoided in young children and after the first dose in adolescents and adults since they do not allow for individualization of charcoal and sorbitol dosing. Ipecac syrup should be avoided in theophylline overdoses. Although ipecac induces emesis, it does not reduce the absorption of theophylline unless administered within 5 minutes of ingestion and even then is less effective than oral activated charcoal. Moreover, ipecac induced emesis may persist for several hours after a single dose and significantly decrease the retention and the effectiveness of oral activated charcoal.
- Serum Theophylline Concentration Monitoring The serum theophylline concentration should be measured immediately upon presentation, 2-4 hours later, and then at sufficient intervals, e.g., every 4 hours, to guide treatment decisions and to assess the effectiveness of therapy. Serum theophylline concentrations may continue to increase after presentation of the patient for medical care as a result of continued absorption of theophylline from the gastrointestinal tract. Serial monitoring of serum theophylline serum concentrations should be continued until it is clear that the concentration is no longer rising and has returned to non-toxic levels.
- General Monitoring Procedures Electrocardiographic monitoring should be initiated on presentation and continued until the serum theophylline level has returned to a non-toxic level. Serum electrolytes and glucose should be measured on presentation and at appropriate intervals indicated by clinical circumstances. Fluid and electrolyte abnormalities should be promptly corrected. Monitoring and treatment should be continued until the serum concentration decreases below 20 mcg/mL.
- Enhance clearance of theophylline Multiple-dose oral activated charcoal (e.g., 0.5 mg/kg up to 20 g, every two hours) increases the clearance of theophylline at least twofold by adsorption of theophylline secreted into gastrointestinal fluids. Charcoal must be retained in, and pass through, the gastrointestinal tract to be effective; emesis should therefore be controlled by administration of appropriate antiemetics. Alternatively, the charcoal can be administered continuously through a nasogastric tube in conjunction with appropriate antiemetics. A single dose of sorbitol may be administered with the activated charcoal to promote stooling to facilitate clearance of the adsorbed theophylline from the gastrointestinal tract. Sorbitol alone does not enhance clearance of theophylline and should be dosed with caution to prevent excessive stooling which can result in severe fluid and electrolyte imbalances. Commercially available fixed combinations of liquid charcoal and sorbitol should be avoided in young children and after the first dose in adolescents and adults since they do not allow for individualization of charcoal and sorbitol dosing. In patients with intractable vomiting, extracorporeal methods of theophylline removal should be instituted (see OVERDOSAGE, Extracorporeal Removal).
Specific Recommendations
Acute Overdose
- A. Serum Concentration >20 <30 mcg/mL
- Administer a single dose of oral activated charcoal.
- Monitor the patient and obtain a serum theophylline concentration in 2-4 hours to insure that the concentration is not increasing.
- B. Serum Concentration >30 <100 mcg/mL
- Administer multiple dose oral activated charcoal and measures to control emesis.
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
- Institute extracorporeal removal if emesis, seizures, or cardiac arrhythmias cannot be adequately controlled (see OVERDOSAGE, Extracorporeal Removal).
- C. Serum Concentration >100 mcg/mL
- Consider prophylactic anticonvulsant therapy.
- Administer multiple-dose oral activated charcoal and measures to control emesis.
- Consider extracorporeal removal, even if the patient has not experienced a seizure (see OVERDOSAGE, Extracorporeal Removal).
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
Chronic Overdosage
- A.
Serum Concentration >20 <30 mcg/mL (with manifestations of theophylline toxicity)
- Administer a single dose of oral activated charcoal.
- Monitor the patient and obtain a serum theophylline concentration in 2-4 hours to insure that the concentration is not increasing.
- B.
Serum Concentration >30 mcg/mL in patients <60 years of age
- Administer multiple-dose oral activated charcoal and measures to control emesis.
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
- Institute extracorporeal removal if emesis, seizures, or cardiac arrhythmias cannot be adequately controlled (see OVERDOSAGE, Extracorporeal Removal).
- C.
Serum Concentration >30 mcg/mL in patients ≥60 years of age.
- Consider prophylactic anticonvulsant therapy.
- Administer multiple-dose oral activated charcoal and measures to control emesis.
- Consider extracorporeal removal even if the patient has not experienced a seizure (see OVERDOSAGE, Extracorporeal Removal).
- Monitor the patient and obtain serial theophylline concentrations every 2-4 hours to gauge the effectiveness of therapy and to guide further treatment decisions.
Extracorporeal Removal
Increasing the rate of theophylline clearance by extracorporeal methods may rapidly decrease serum concentrations, but the risks of the procedure must be weighed against the potential benefit. Charcoal hemoperfusion is the most effective method of extracorporeal removal, increasing theophylline clearance up to sixfold, but serious complications, including hypotension, hypocalcemia, platelet consumption and bleeding diatheses may occur. Hemodialysis is about as efficient as multiple-dose oral activated charcoal and has a lower risk of serious complications than charcoal hemoperfusion. Hemodialysis should be considered as an alternative when charcoal hemoperfusion is not feasible and multiple-dose oral charcoal is ineffective because of intractable emesis. Serum theophylline concentrations may rebound 5-10 mcg/mL after discontinuation of charcoal hemoperfusion or hemodialysis due to redistribution of theophylline from the tissue compartment. Peritoneal dialysis is ineffective for theophylline removal; exchange transfusions in neonates have been minimally effective.
-
DOSAGE AND ADMINISTRATION
Theophylline (Anhydrous) Extended-Release Tablets 400 or 600 mg can be taken once a day in the morning or evening. It is recommended that Theophylline (Anhydrous) Extended-Release Tablets be taken with meals. Patients should be advised that if they choose to take Theophylline (Anhydrous) Extended-Release Tablets with food it should be taken consistently with food and if they take it in a fasted condition it should routinely be taken fasted. It is important that the product whenever dosed be dosed consistently with or without food.
Theophylline (Anhydrous) Extended-Release Tablets are not to be chewed or crushed because it may lead to a rapid release of theophylline with the potential for toxicity. The scored tablet may be split. Infrequently, patients receiving Theophylline (Anhydrous) Extended-Release Tablets 400 or 600 mg may pass an intact matrix tablet in the stool or via colostomy. These matrix tablets usually contain little or no residual theophylline.
Stabilized patients, 12 years of age or older, who are taking an immediate-release or controlled-release theophylline product may be transferred to once-daily administration of 400 or 600 mg Theophylline (Anhydrous) Extended-Release Tablets on a mg-for-mg basis.
It must be recognized that the peak and trough serum theophylline levels produced by the once-daily dosing may vary from those produced by the previous product and/or regimen.
General Considerations
The steady-state peak serum theophylline concentration is a function of the dose, the dosing interval, and the rate of theophylline absorption and clearance in the individual patient. Because of marked individual differences in the rate of theophylline clearance, the dose required to achieve a peak serum theophylline concentration in the 10-20 mcg/mL range varies fourfold among otherwise similar patients in the absence of factors known to alter theophylline clearance (e.g., 400‑1600 mg/day in adults <60 years old and 10-36 mg/kg/day in children 1-9 years old). For a given population there is no single theophylline dose that will provide both safe and effective serum concentrations for all patients. Administration of the median theophylline dose required to achieve a therapeutic serum theophylline concentration in a given population may result in either sub-therapeutic or potentially toxic serum theophylline concentrations in individual patients. For example, at a dose of 900 mg/d in adults <60 years or 22 mg/kg/d in children 1-9 years, the steady-state peak serum theophylline concentration will be <10 mcg/mL in about 30% of patients, 10-20 mcg/mL in about 50% and 20-30 mcg/mL in about 20% of patients. The dose of theophylline must be individualized on the basis of peak serum theophylline concentration measurements in order to achieve a dose that will provide maximum potential benefit with minimal risk of adverse effects.
Transient caffeine-like adverse effects and excessive serum concentrations in slow metabolizers can be avoided in most patients by starting with a sufficiently low dose and slowly increasing the dose, if judged to be clinically indicated, in small increments (See Table V). Dose increases should only be made if the previous dosage is well tolerated and at intervals of no less than 3 days to allow serum theophylline concentrations to reach the new steady-state. Dosage adjustment should be guided by serum theophylline concentration measurement (see PRECAUTIONS, Laboratory Tests and DOSAGE AND ADMINISTRATION, Table VI). Healthcare providers should instruct patients and caregivers to discontinue any dosage that causes adverse effects, to withhold the medication until these symptoms are gone and to then resume therapy at a lower, previously tolerated dosage (see WARNINGS).
If the patient's symptoms are well controlled, there are no apparent adverse effects, and no intervening factors that might alter dosage requirements (see WARNINGS and PRECAUTIONS), serum theophylline concentrations should be monitored at 6 month intervals for rapidly growing children and at yearly intervals for all others. In acutely ill patients, serum theophylline concentrations should be monitored at frequent intervals, e.g., every 24 hours.
Theophylline distributes poorly into body fat, therefore, mg/kg dose should be calculated on the basis of ideal body weight.
Table V contains theophylline dosing titration schema recommended for patients in various age groups and clinical circumstances. Table VI contains recommendations for theophylline dosage adjustment based upon serum theophylline concentrations. Application of these general dosing recommendations to individual patients must take into account the unique clinical characteristics of each patient. In general, these recommendations should serve as the upper limit for dosage adjustments in order to decrease the risk of potentially serious adverse events associated with unexpected large increases in serum theophylline concentration.
Table V. Dosing initiation and titration (as anhydrous theophylline).1
A. Children (12-15 years) and adults (16-60 years) without risk factors for impaired clearance.
Titration Step Children <45 kg Children >45 kg and adults - * If caffeine-like adverse effects occur, then consideration should be given to a lower dose and titrating the dose more slowly (see ADVERSE REACTIONS).
- 1. Starting Dosage
12-14 mg/kg/day up to a maximum of 300 mg/day admin. QD1 300 mg/day* admin. QD1 - 2. After 3 days, if tolerated, increase dose to:
16 mg/kg/day up to a maximum of 400 mg/day admin. QD1 400-600 mg/day* admin. QD1 - 3. After 3 more days, if tolerated, and if needed increase dose to:
20 mg/kg/day up to a maximum of 600 mg/day admin. QD1 As with all theophylline products, doses greater than 600 mg should be titrated according to blood level (See Table VI). B. Patients With Risk Factors For Impaired Clearance, The Elderly (>60 Years), And Those In Whom It Is Not Feasible To Monitor Serum Theophylline Concentrations:
In children 12-15 years of age, the theophylline dose should not exceed 16 mg/kg/day up to a maximum of 400 mg/day in the presence of risk factors for reduced theophylline clearance (see WARNINGS) or if it is not feasible to monitor serum theophylline concentrations.
In adolescents ≥16 years and adults, including the elderly, the theophylline dose should not exceed 400 mg/day in the presence of risk factors for reduced theophylline clearance (see WARNINGS) or if it is not feasible to monitor serum theophylline concentrations.
- 1 Patients with more rapid metabolism clinically identified by higher than average dose requirements, should receive a smaller dose more frequently (every 12 hours) to prevent breakthrough symptoms resulting from low trough concentrations before the next dose.
Table VI. Dosage adjustment guided by serum theophylline concentration. Peak Serum Concentration Dosage Adjustment - * Dose reduction and/or serum theophylline concentration measurement is indicated whenever adverse effects are present physiologic abnormalities that can reduce theophylline clearance occur (e.g., sustained fever), or a drug that interacts with theophylline is added or discontinued (see WARNINGS).
<9.9 mcg/mL If symptoms are not controlled and current dosage is tolerated, increase dose about 25%.
Recheck serum concentration after three days for further dosage adjustment.10-14.9 mcg/mL If symptoms are controlled and current dosage is tolerated, maintain dose and recheck serum concentration at 6-12 month intervals.* If symptoms are not controlled and current dosage is tolerated consider adding additional medication(s) to treatment regimen. 15-19.9 mcg/mL Consider 10% decrease in dose to provide greater margin of safety even if current dosage is tolerated.* 20-24.9 mcg/mL Decrease dose by 25% even if no adverse effects are present. Recheck serum concentration after
3 days to guide further dosage adjustment.25-30 mcg/mL Skip next dose and decrease subsequent doses at least 25% even if no adverse effects are present. Recheck serum concentration after 3 days to guide further dosage adjustment. If symptomatic, consider whether overdose treatment is indicated (see recommendations for chronic overdosage). >30 mcg/mL Treat overdose as indicated (see recommendations for chronic overdosage). If theophylline is subsequently resumed, decrease dose by at least 50% and recheck serum concentration after 3 days to guide further dosage adjustment. -
HOW SUPPLIED
Theophylline (Anhydrous) Extended-Release Tablets 400 mg are supplied as white, round, bisected tablets debossed with "N" above the bisect and "T4" below the bisect, available in bottles of 100 tablets (NDC: 69367-414-01).
Theophylline (Anhydrous) Extended-Release Tablets 600 mg are supplied as white, oblong, bisected tablets on one side and debossed with "NT6" on the other side, available in bottles of 100 tablets (NDC: 69367-415-01).
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label
NDC: 69367-414-01
Rx OnlyTheophylline (Anhydrous)
Extended-Release Tablets400 mg
100 Tablets
Westminster
Pharmaceuticals
-
PRINCIPAL DISPLAY PANEL - 600 mg Tablet Bottle Label
NDC: 69367-415-01
Rx OnlyTheophylline (Anhydrous)
Extended-Release Tablets600 mg
100 Tablets
Westminster
Pharmaceuticals
-
INGREDIENTS AND APPEARANCE
THEOPHYLLINE (ANHYDROUS)
theophylline anhydrous tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69367-414 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THEOPHYLLINE ANHYDROUS (UNII: 0I55128JYK) (THEOPHYLLINE ANHYDROUS - UNII:0I55128JYK) THEOPHYLLINE ANHYDROUS 400 mg Inactive Ingredients Ingredient Name Strength GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 11mm Flavor Imprint Code N;T4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69367-414-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/25/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040560 11/25/2025 THEOPHYLLINE (ANHYDROUS)
theophylline anhydrous tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69367-415 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THEOPHYLLINE ANHYDROUS (UNII: 0I55128JYK) (THEOPHYLLINE ANHYDROUS - UNII:0I55128JYK) THEOPHYLLINE ANHYDROUS 600 mg Inactive Ingredients Ingredient Name Strength GLYCERYL BEHENATE/EICOSADIOATE (UNII: 73CJJ317SR) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE Score 2 pieces Shape OVAL (oblong) Size 19mm Flavor Imprint Code NT6 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69367-415-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/25/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040560 11/25/2025 Labeler - Westminster Pharmaceuticals, LLC (079516651) Registrant - MPP Pharma LLC (137555154) Establishment Name Address ID/FEI Business Operations MPP Pharma LLC 137555154 ANALYSIS(69367-414, 69367-415) , MANUFACTURE(69367-414, 69367-415) , PACK(69367-414, 69367-415) , LABEL(69367-414, 69367-415)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.