MOXEZA- moxifloxacin hydrochloride solution
MOXEZA by
Drug Labeling and Warnings
MOXEZA by is a Prescription medication manufactured, distributed, or labeled by Alcon Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MOXEZA® solution safely and effectively. See full prescribing information for MOXEZA®.
MOXEZA® (moxifloxacin ophthalmic solution) 0.5%
Sterile topical ophthalmic solution
Initial U.S. Approval: 1999INDICATIONS AND USAGE
MOXEZA® solution is a topical fluoroquinolone anti-infective indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Aerococcus viridans*, Corynebacterium macginleyi*, Enterococcus faecalis*, Micrococcus luteus*, Staphylococcus arlettae*, Staphylococcus aureus, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus saprophyticus*, Staphylococcus warneri*, Streptococcus mitis*, Streptococcus pneumoniae, Streptococcus parasanguinis*, Escherichia coli*, Haemophilus influenzae, Klebsiella pneumoniae*, Propionibacterium acnes, Chlamydia trachomatis*
*Efficacy for this organism was studied in fewer than 10 infections. (1)DOSAGE AND ADMINISTRATION
Instill 1 drop in the affected eye(s) 2 times daily for 7 days. (2)
DOSAGE FORMS AND STRENGTHS
4 mL bottle filled with 3 mL sterile ophthalmic solution of moxifloxacin 0.5%. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Topical ophthalmic use only (5.1)
- Hypersensitivity and anaphylaxis have been reported with systemic use of moxifloxacin (5.2)
- Prolonged use may result in overgrowth of non-susceptible organisms, including fungi (5.3)
- Patients should not wear contact lenses if they have signs or symptoms of bacterial conjunctivitis. (5.4)
ADVERSE REACTIONS
The most common adverse reactions reported in 1-2% of patients were eye irritation, pyrexia, and conjunctivitis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories, Inc. at 1-800-757-9195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
5.2 Hypersensitivity Reactions
5.3 Growth of Resistant Organisms with Prolonged Use
5.4 Avoidance of Contact Lens Wear
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Avoid Contamination of the Product
17.2 Avoid Contact Lens Wear
17.3 Hypersensitivity Reactions
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
MOXEZA® solution is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Aerococcus viridans*
Corynebacterium macginleyi*
Enterococcus faecalis*
Micrococcus luteus*
Staphylococcus arlettae*
Staphylococcus aureus
Staphylococcus capitis
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus saprophyticus*
Staphylococcus warneri*
Streptococcus mitis*
Streptococcus pneumoniae
Streptococcus parasanguinis*
Escherichia coli*
Haemophilus influenza
Klebsiella pneumoniae*
Propionibacterium acnes
Chlamydia trachomatis*
*Efficacy for this organism was studied in fewer than 10 infections. - 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
NOT FOR INJECTION. MOXEZA® solution is for topical ophthalmic use only and should not be injected subconjunctivally or introduced directly into the anterior chamber of the eye.
5.2 Hypersensitivity Reactions
In patients receiving systemically administered quinolones, including moxifloxacin, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported, some following the first dose. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. If an allergic reaction to moxifloxacin occurs, discontinue use of the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management should be administered as clinically indicated.
5.3 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and, where appropriate, fluorescein staining.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to MOXEZA® solution in 1263 patients, between 4 months and 92 years of age, with signs and symptoms of bacterial conjunctivitis. The most frequently reported adverse reactions were eye irritation, pyrexia and conjunctivitis, reported in 1-2% of patients.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Moxifloxacin was not teratogenic when administered to pregnant rats during organogenesis at oral doses as high as 500 mg/kg/day (approximately 25,000 times the highest recommended total daily human ophthalmic dose); however, decreased fetal body weights and slightly delayed fetal skeletal development were observed. There was no evidence of teratogenicity when pregnant Cynomolgus monkeys were given oral doses as high as 100 mg/kg/day (approximately 5,000 times the highest recommended total daily human ophthalmic dose). An increased incidence of smaller fetuses was observed at 100 mg/kg/day.
Since there are no adequate and well-controlled studies in pregnant women, MOXEZA® solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Moxifloxacin has not been measured in human milk, although it can be presumed to be excreted in human milk. Caution should be exercised when MOXEZA® solution is administered to a nursing mother.
8.4 Pediatric Use
The safety and effectiveness of MOXEZA® solution in infants below 4 months of age have not been established.
There is no evidence that the ophthalmic administration of moxifloxacin has any effect on weight bearing joints, even though oral administration of some quinolones has been shown to cause arthropathy in immature animals.
-
11 DESCRIPTION
MOXEZA® is a sterile solution for topical ophthalmic use.
Moxifloxacin hydrochloride is an 8-methoxy fluoroquinolone anti-infective, with a diazabicyclononyl ring at the C7 position.
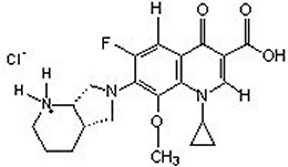
C21H24FN3O4HCl Mol Wt 437.9
Chemical Name: 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolol[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid, monohydrochloride.
Each mL of MOXEZA® solution contains 5.45 mg moxifloxacin hydrochloride, equivalent to 5 mg moxifloxacin base.
Inactives: Sodium chloride, xanthan gum, boric acid, sorbitol, tyloxapol, purified water, and hydrochloric acid and/or sodium hydroxide to adjust pH.
MOXEZA® is a greenish-yellow, isotonic solution with an osmolality of 300-370 mOsm/kg and a pH of approximately 7.4. Moxifloxacin hydrochloride is a slightly yellow to yellow crystalline powder.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Moxifloxacin is a member of the fluoroquinolone class of anti-infective drugs [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
Moxifloxacin steady-state plasma pharmacokinetics were evaluated in healthy adult male and female subjects who were administered multiple, bilateral, topical ocular doses of MOXEZA® solution two times daily for four days with a final dose on day 5. The average steady-state AUC0-12 was 8.17 ± 5.31 ng*h/mL. Moxifloxacin Cmax following twice-daily bilateral ophthalmic administration of moxifloxacin 0.5% for 5 days is approximately 0.02% of that achieved with the oral formulation of moxifloxacin hydrochloride (Cmax following oral dosing of 400 mg AVELOX*, 4.5 ± 0.5 mcg/mL).
12.4 Microbiology
The antibacterial action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division.
The mechanism of action for quinolones, including moxifloxacin, is different from that of macrolides, aminoglycosides, or tetracyclines. Therefore, moxifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to moxifloxacin. There is no cross-resistance between moxifloxacin and the aforementioned classes of antibiotics. Cross-resistance has been observed between systemic moxifloxacin and some other quinolones.
In vitro resistance to moxifloxacin develops via multiple-step mutations. Resistance to moxifloxacin occurs in vitro at a general frequency of between 1.8 x 10-9 to < 1x10-11 for Gram-positive bacteria.
Moxifloxacin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Aerococcus viridans*
Corynebacterium macginleyi*
Enterococcus faecalis*
Micrococcus luteus*
Staphylococcus arlettae*
Staphylococcus aureus
Staphylococcus capitis
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus saprophyticus*
Staphylococcus warneri*
Streptococcus mitis*
Streptococcus pneumoniae
Streptococcus parasanguinis*
Escherichia coli*
Haemophilus influenza
Klebsiella pneumoniae*
Propionibacterium acnes
Chlamydia trachomatis**Efficacy for this organism was studied in fewer than 10 infections.
The following in vitro data are available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of MOXEZA® solution in treating ophthalmic infections due to these organisms have not been established in adequate and well-controlled trials.
Moxifloxacin has been shown to be active in vitro against most strains of the microorganisms listed below. These organisms are considered susceptible when evaluated using systemic breakpoints; however, a correlation between the in vitro systemic breakpoint and ophthalmologic efficacy has not been established. The list of organisms is provided as guidance only in assessing the potential treatment of conjunctival infections. Moxifloxacin exhibits in vitro minimal inhibitory concentrations (MICs) of 2 mcg/mL or less (systemic susceptible breakpoint) against most (≥ 90%) strains of the following ocular pathogens.
Aerobic Gram-positive microorganisms:
Staphylococcus caprae
Staphylococcus cohnii
Staphylococcus lugdunensis
Staphylococcus pasteuri
Streptococcus agalactiae
Streptococcus milleri group
Streptococcus oralis
Streptococcus pyogenes
Streptococcus salivarius
Streptococcus sanguisAerobic Gram-negative microorganisms:
Acinetobacter baumannii
Acinetobacter calcoaceticus
Acinetobacter junii
Enterobacter aerogenes
Enterobacter cloacae
Haemophilus parainfluenzae
Klebsiella oxytoca
Moraxella catarrhalis
Moraxella osloensis
Morganella morganii
Neisseria gonorrhoeae
Neisseria meningitides
Pantoea agglomerans
Proteus vulgaris
Pseudomonas stutzeri
Serratia liquefaciens
Serratia marcescens
Stenotrophomonas maltophiliaAnaerobic microorganisms:
Clostridium perfringens
Peptostreptococcus anaerobius
Peptostreptococcus magnus
Peptostreptococcus micros
Peptostreptococcus prevotiiOther microorganisms:
Mycobacterium tuberculosis
Mycobacterium avium
Mycobacterium kansasii
Mycobacterium marinum -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to determine the carcinogenic potential of moxifloxacin have not been performed.
Moxifloxacin was not mutagenic in four bacterial strains used in the Ames Salmonella reversion assay. As with other quinolones, the positive response observed with moxifloxacin in strain TA 102 using the same assay may be due to the inhibition of DNA gyrase. Moxifloxacin was not mutagenic in the CHO/HGPRT mammalian cell gene mutation assay. An equivocal result was obtained in the same assay when v79 cells were used. Moxifloxacin was clastogenic in the v79 chromosome aberration assay, but it did not induce unscheduled DNA synthesis in cultured rat hepatocytes. There was no evidence of genotoxicity in vivo in a micronucleus test or a dominant lethal test in mice.
Moxifloxacin had no effect on fertility in male and female rats at oral doses as high as 500 mg/kg/day, approximately 25,000 times the highest recommended total daily human ophthalmic dose. At 500 mg/kg orally there were slight effects on sperm morphology (head-tail separation) in male rats and on the estrous cycle in female rats.
-
14 CLINICAL STUDIES
In one randomized, double-masked, multicenter, vehicle-controlled clinical trial in which patients with bacterial conjunctivitis were dosed with MOXEZA® solution 2 times a day, MOXEZA® was superior to its vehicle for both clinical and microbiological outcomes. Clinical cure achieved on Day 4 was 63% (265/424) in MOXEZA® solution treated patients, versus 51% (214/423) in vehicle treated patients. Microbiologic success (eradication of baseline pathogens) was achieved on Day 4 in 75% (316/424) of MOXEZA® solution treated patients versus 56% (237/423) of vehicle treated patients. Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
MOXEZA® solution is supplied as a sterile ophthalmic solution in the Alcon DROP-TAINER® dispensing system consisting of a natural low density polyethylene bottle and dispensing plug and tan polypropylene closure. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
3 mL in a 4 mL bottle - NDC: 0065-0006-03
Storage: Store at 2°C - 25°C (36°F - 77°F).
-
17 PATIENT COUNSELING INFORMATION
17.1 Avoid Contamination of the Product
Advise patients not to touch the dropper tip to any surface to avoid contaminating the contents.
17.2 Avoid Contact Lens Wear
Advise patients not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis.
17.3 Hypersensitivity Reactions
Systemically administered quinolones, including moxifloxacin, have been associated with hypersensitivity reactions, even following a single dose. Patients should be told to discontinue use immediately and contact their physician at the first sign of a rash or allergic reaction.
Licensed to Alcon by Bayer Pharma AG.
©2010, 2012, 2016 Novartis
*AVELOX is a registered trademark of Bayer AG.
Alcon ®
a Novartis company
Distributed by:
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134 USA
alcon.medinfo@alcon.com
T2017-23
February 2017 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MOXEZA
moxifloxacin hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0065-0006 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIFLOXACIN HYDROCHLORIDE (UNII: C53598599T) (MOXIFLOXACIN - UNII:U188XYD42P) MOXIFLOXACIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) XANTHAN GUM (UNII: TTV12P4NEE) BORIC ACID (UNII: R57ZHV85D4) SORBITOL (UNII: 506T60A25R) TYLOXAPOL (UNII: Y27PUL9H56) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0065-0006-03 3 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022428 01/18/2011 Labeler - Alcon Laboratories, Inc. (008018525)
Trademark Results [MOXEZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MOXEZA 85032907 3928077 Live/Registered |
NOVARTIS AG 2010-05-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
