AVON SUN SUNSCREEN- homosalate, oxybenzone, octisalate, avobenzone, octocrylene lotion
Avon Sun by
Drug Labeling and Warnings
Avon Sun by is a Otc medication manufactured, distributed, or labeled by New Avon LLC, Avon Products, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
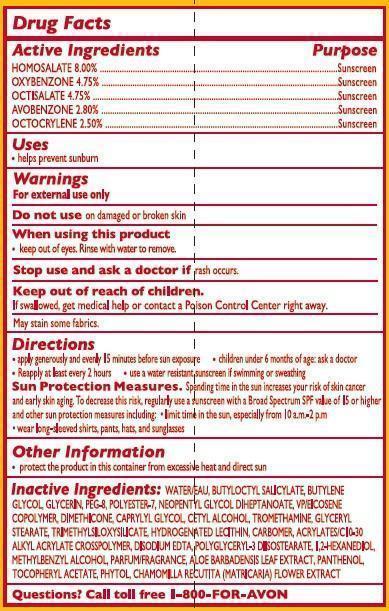
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
apply generously and evenly 15 minutes before sun exposure
children under 6 months of age: ask a doctorreapply after 80 minutes of swimming or sweating
immediately after towel dryingat least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
limit time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses - STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive Ingredients:
WATER/EAU, BUTYLOCTYL SALICYLATE, BUTYLENE GLYCOL, GLYCERIN, PEG-8, POLYESTER-7, NEOPENTYL GLYCOL DIHEPTANOATE, VP/EICOSENE COPOLYMER, DIMETHICONE, CAPRYLYL GLYCOL, CETYL ALCOHOL, TROMETHAMINE, GLYCERYL STEARATE, TRIMETHYLSILOXYSILICATE, HYDROGENATED LECITHIN, CARBOMER, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, DISODIUM EDTA,
POLYGLYCERYL-3 DIISOSTEARATE, 1,2-HEXANEDIOL, METHYLBENZYL ALCOHOL, PARFUM/FRAGRANCE, ALOE BARBADENSIS LEAF EXTRACT, PANTHENOL, TOCOPHERYL ACETATE, PHYTOL, CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT, - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AVON SUN SUNSCREEN
homosalate, oxybenzone, octisalate, avobenzone, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10096-0295 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 80 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 47.5 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 47.5 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 28 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10096-0295-1 236 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/26/2013 Labeler - New Avon LLC (080143520) Establishment Name Address ID/FEI Business Operations Avon Products, Inc 005149471 manufacture(10096-0295)
Trademark Results [Avon Sun]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AVON SUN 75509482 2253734 Dead/Cancelled |
Avon Products, Inc. 1998-06-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.