SINECCH (CANADA)- arnica montana capsule
SinEcch (Canada) by
Drug Labeling and Warnings
SinEcch (Canada) by is a Otc medication manufactured, distributed, or labeled by Hahnemann Laboratories, INC dba Alpine Pharmaceuticals, Hahnemann Laboratories, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- PATIENT MEDICATION INFORMATION
- ACTIVE INGREDIENT
- DOSAGE FORMS & STRENGTHS
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS AND PRECAUTIONS
- DO NOT USE
- DRUG INTERACTIONS
- ASK DOCTOR
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INSTRUCTIONS FOR USE
- GENERAL PRECAUTIONS
- ROUTE, METHOD AND FREQUENCY OF ADMINISTRATION
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
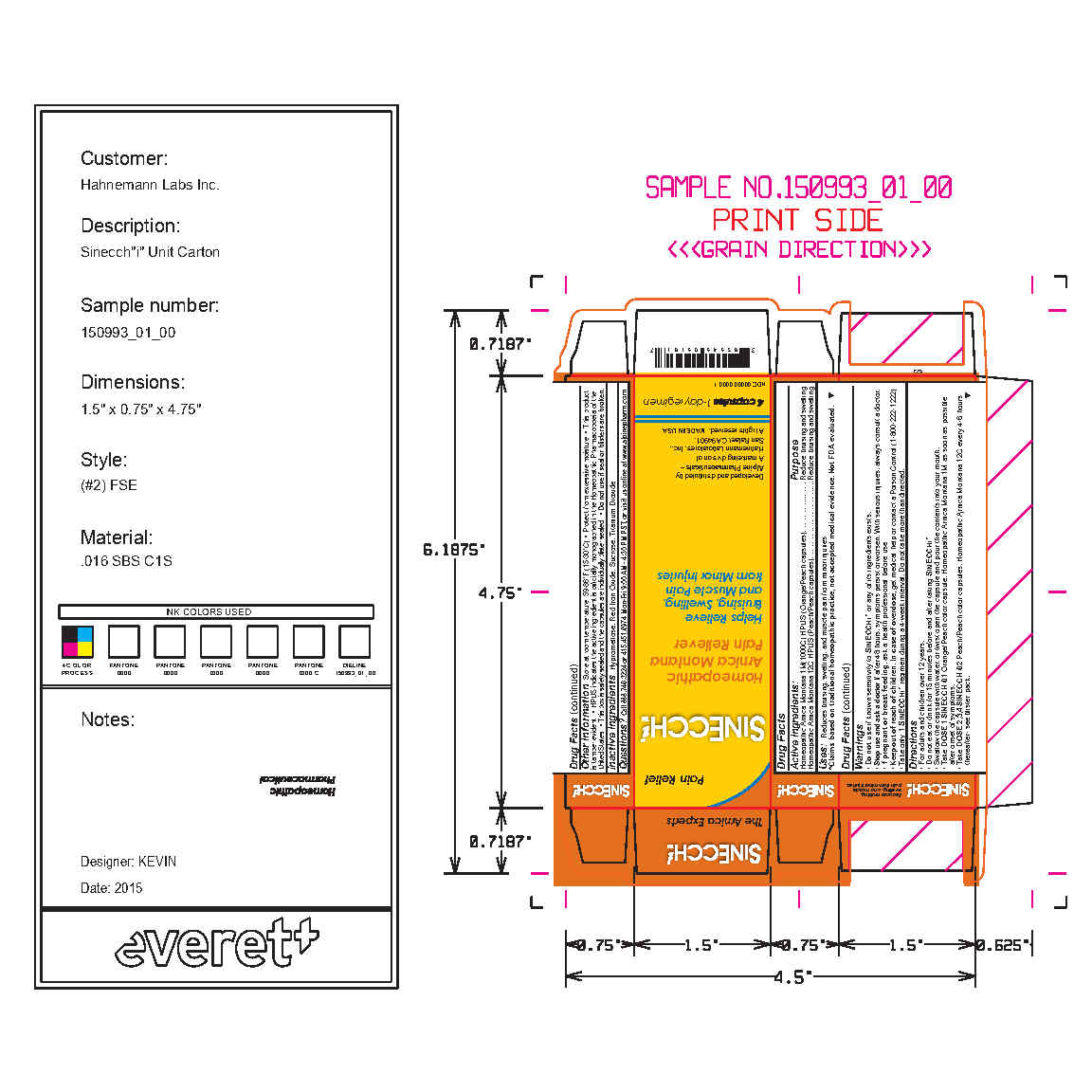
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SINECCH (CANADA)
arnica montana capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37662-2085 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_M] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color orange (Orange/ Peach (1M Strength)) , pink (Peach Capsules (12C Strength)) Score no score Shape CAPSULE Size 20mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37662-2085-3 16 in 1 BOX 09/19/2023 1 NDC: 37662-2085-2 1 in 1 BOX 1 NDC: 37662-2085-1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 09/19/2023 Labeler - Hahnemann Laboratories, INC dba Alpine Pharmaceuticals (147098081) Establishment Name Address ID/FEI Business Operations Hahnemann Laboratories, INC 147098081 manufacture(37662-2085)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.