SODIUM CHLORIDE irrigant
Sodium Chloride by
Drug Labeling and Warnings
Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
0.9% Sodium Chloride Irrigation, USP is a sterile nonpyrogenic, isotonic solution in a single dose ARTHROMATIC plastic container for use as an arthroscopic irrigating solution. Each liter contains 9 g Sodium Chloride, USP (NaCl) in Water for Injection. pH 5.5 (4.5 to 7.0). Milliequivalents per liter: Sodium - 154, Chloride - 154. Osmolarity 308 mOsmol/L (calc.). No antimicrobial agent has been added.
The ARTHROMATIC plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexylphthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
-
CLINICAL PHARMACOLOGY
0.9% Sodium Chloride Irrigation, USP is useful as an irrigating fluid for body joints because the solution is isotonic, and provides a transparent fluid medium with optical properties suitable for good visualization of the interior joint surface during endoscopic examination. During arthroscopic surgical procedures, the solution acts as a lavage for removing blood, tissue fragments, and bone fragments.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Not for injection.
Because fluids used to irrigate joints may be absorbed into the general circulation, solutions containing sodium ion should be used with great care in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.
The contents of an opened container should be used promptly to minimize the possibility of bacterial growth or pyrogen formation. Discard the unused portion of irrigating solution since no antimicrobial agent has been added.
- PRECAUTIONS
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
The volume of solution needed will vary with the nature and duration of the arthroscopic procedure.
If desired, warm in overpouch to near body temperature in a water bath or oven heated to not more than 45º C.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
-
HOW SUPPLIED
0.9% Sodium Chloride Irrigation, USP in ARTHROMATIC Plastic Container is available as follows:
2B7477
3000 mL
NDC: 0338-0047-27
2B7479
5000 mL
NDC: 0338-0047-29
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25º C); brief exposure up to 40º C does not adversely affect the product.
-
DIRECTIONS FOR USE
Tear overwrap down side at slit and remove solution container. Visually inspect the container. If the outlet port protector is damaged, detached, or not present, discard container as solution path sterility may be impaired. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing bag firmly. If leaks are found, discard solution as sterility may be impaired.
Use Aseptic Technique.
- 1. Suspend container using hanger hole.
- 2. Remove protector from outlet port.
- 3. Attach irrigation set. Refer to complete directions accompanying set.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
07-19-00-0407
Rev. July 2018Baxter and Arthromatic are trademarks of Baxter International Inc.
-
PACKAGE LABEL.PRINCIPLE DISPLAY PANEL
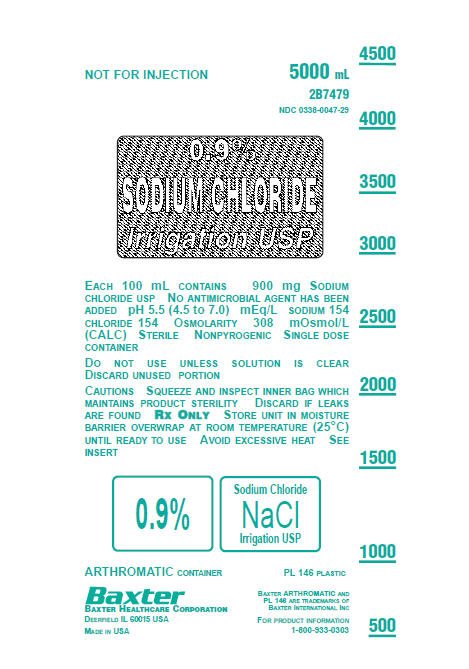
0.9% Sodium Chloride Irrigation USP Container Label
NOT FOR INJECTION
5000 mL
2B7479
NDC: 0338-0047-29
0.9% SODIUM CHLORIDE Irrigation USP
EACH 100 mL CONTAINS 900 mg SODIUM
CHLORIDE USP NO ANTIMICROBIAL AGENT HAS BEEN
ADDED pH 5.5 (4.5 to 7.0) mEq/L SODIUM 154
CHLORIDE 154 OSMOLARITY 308 mOsmol/L
(CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER
DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION
CAUTIONS SQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS
ARE FOUND RX ONLY STORE UNIT IN MOISTURE
BARRIER OVERWRAP AT ROOM TEMPERATURE (25°C)
UNTIL READY TO USE AVOID EXCESSIVE HEAT SEE
INSERT
0.9% Sodium Chloride NaCl Irrigation USP
ARTHROMATIC CONTAINER PL 146 PLASTIC
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA
BAXTER ARTHROMATIC AND
PL 146 ARE TRADEMARKS OF
BAXTER INTERNATIONAL INC
FOR PRODUCT INFORMATION
1-800-933-0303
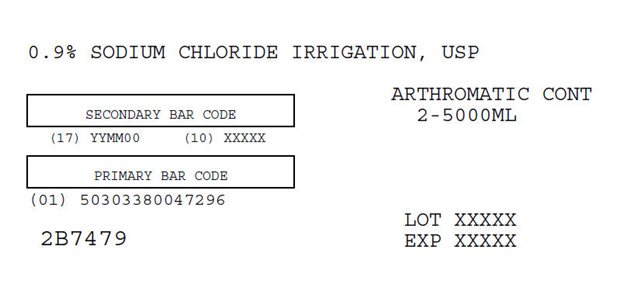
0.9% Sodium Chloride Irrigation USP Carton Label
0.9% SODIUM CHLORIDE IRRIGATION, USP
ARTHROMATIC CONT
2-5000ML
SECONDARY BAR CODE
(17) YYMM00 (10) XXXXX
PRIMARY BAR CODE
(01) 50303380047296
LOT XXXXX
EXP XXXXX
2B7479
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride irrigantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-0047 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 900 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-0047-24 14 in 1 CARTON 05/30/1980 12/22/2012 1 1000 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC: 0338-0047-27 4 in 1 CARTON 05/30/1980 2 3000 mL in 1 BAG; Type 0: Not a Combination Product 3 NDC: 0338-0047-29 2 in 1 CARTON 05/30/1980 3 5000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017867 05/30/1980 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 059140764 ANALYSIS(0338-0047) , LABEL(0338-0047) , MANUFACTURE(0338-0047) , PACK(0338-0047) , STERILIZE(0338-0047) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-0047)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.