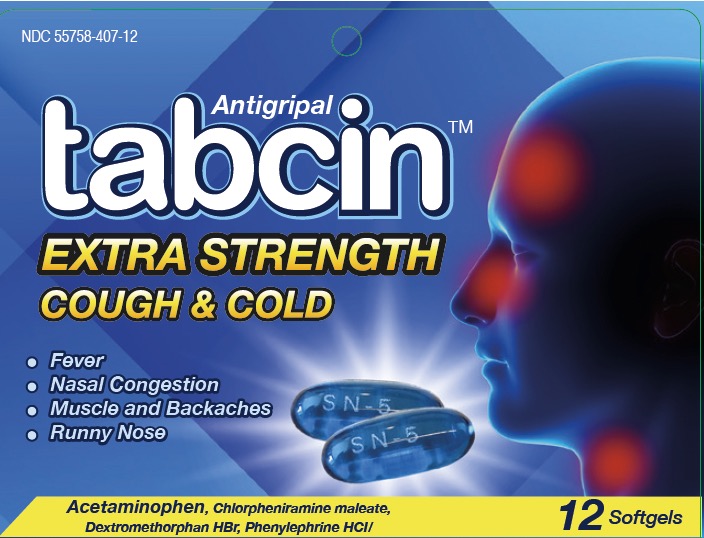
TABCIN EXTRA STRENGTH- acetaminophen, chlorpheniramine maleate, dextromethorphan hbr, phenylephrine hcl, capsule, liquid filled
Tabcin by
Drug Labeling and Warnings
Tabcin by is a Otc medication manufactured, distributed, or labeled by Pharmadel LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
-
Active Ingredients & Purposes
Acetaminophen 250mg
Chlorpheniramine maleate 2 mg
Dextromethorphan hydrobromide 10 mg
Phenylephrine hydrochloride 5 mg
Active Ingredients Purposes Acetaminophen 250mg................................... Pain reliever/ fever reducer Chlorpheniramine maleate 2 mg.................... Antihistamine Dextromethorphan hydrobromide 10 mg........ Cough suppressant Phenylephrine hydrochloride 5 mg.................. Nasal decongestant - Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 10 softgels in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert:
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning:
If sore throat is severe, persists for more than 2 days, and is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- a persistent or chronic cough such as occurs with smoking, asthma, or emphysema, or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed the recommended dosage
- may cause excitability, especially in children
- may cause drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effect avoid alcoholic beverages while taking this product
- use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occurs
- the pain or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with a rash or headache that lasts.
These could be signs of a serious condition.
- Directions
- Other information
- Inactive ingredients
- Questions or comments
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TABCIN EXTRA STRENGTH
acetaminophen, chlorpheniramine maleate, dextromethorphan hbr, phenylephrine hcl, capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55758-407 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Product Characteristics Color blue Score score with uneven pieces Shape CAPSULE (Oblong) Size 21mm Flavor Imprint Code SN5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55758-407-12 1 in 1 CARTON 10/02/2023 08/01/2027 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/02/2023 08/01/2027 Labeler - Pharmadel LLC (030129680)
Trademark Results [Tabcin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TABCIN 98185587 not registered Live/Pending |
Pharmadel, LLC 2023-09-18 |
 TABCIN 97770548 not registered Live/Pending |
Medimex US 2023-01-27 |
 TABCIN 77742397 4292594 Live/Registered |
Zadik, F. Allan 2009-05-21 |
 TABCIN 71591502 0536762 Dead/Expired |
MILES LABORATORIES, INC. 1950-01-26 |
 TABCIN 71345716 0312953 Dead/Expired |
LECHTZIN, JACOB 1934-01-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.