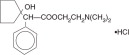CYCLOPENTOLATE HYDROCHLORIDE solution/ drops
Cyclopentolate Hydrochloride by
Drug Labeling and Warnings
Cyclopentolate Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Akorn. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Cyclopentolate Hydrochloride Ophthalmic Solution is an anticholinergic prepared as a sterile, borate buffered solution for topical ocular use. It is supplied in two strengths.
Chemical name:
2-(Dimethylamino)ethyl 1-hydroxy-α-phenylcyclopentaneacetate hydrochloride
MW=327.85 C17H25NO3 ⋅ HCl
The active ingredient is represented by the structural formula:
Cyclopentolate Hydrochloride Ophthalmic Solution USP, 1% & 2%
Each mL contains: Active: Cyclopentolate Hydrochloride 10 mg (1%) or 20 mg (2%).
Inactives: Boric Acid, Edetate Disodium, Potassium Chloride (except 2% strength), Sodium Carbonate and/or Hydrochloric Acid may be added to adjust pH (3.0 to 5.5) and Water for Injection.
Preservative: Benzalkonium Chloride 0.1 mg (0.01%).
-
CLINICAL PHARMACOLOGY:
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia). It acts rapidly, but has a shorter duration than atropine. Maximal cycloplegia occurs within 25 to 75 minutes after instillation. Complete recovery of accommodation usually takes 6 to 24 hours. Complete recovery from mydriasis in some individuals may require several days. Heavily pigmented irides may require more doses than lightly pigmented irides.
- INDICATIONS AND USAGE:
- CONTRAINDICATIONS:
-
WARNINGS:
For topical ophthalmic use only. Not for injection. This preparation may cause CNS disturbances. This is especially true in younger age groups, but may occur at any age, especially with the stronger solutions. Infants are especially prone to CNS and cardiopulmonary side effects from cyclopentolate. To minimize absorption, use only 1 drop of 0.5% cyclopentolate hydrochloride ophthalmic solution per eye, followed by pressure applied over the nasolacrimal sac for two to three minutes. Observe infants closely for at least 30 minutes.
Mydriatics may produce a transient elevation of intraocular pressure.
-
PRECAUTIONS:
General: The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption. Caution should be observed when considering use of this medication in the presence of Down's syndrome and in those predisposed to angle-closure glaucoma.
Information for Patients: Do not touch dropper tip to any surface, as this may contaminate the solution. A transient burning sensation may occur upon instillation. Patients should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patients may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld for four (4) hours after examination.
Drug Interactions: Cyclopentolate may interfere with the ocular anti-hypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors.
Carcinogenesis, Mutagenesis, and Impairment of Fertility: Studies in animals or humans have not been conducted to evaluate the carcinogenic potential of cyclopentolate.
Pregnancy: Pregnancy Category C. Animal reproduction studies have not been conducted with cyclopentolate. It is also not known whether cyclopentolate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Cyclopentolate should be administered to a pregnant woman only if clearly needed.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when cyclopentolate hydrochloride is administered to a nursing woman.
Pediatric Use: Use of cyclopentolate has been associated with psychotic reactions and behavioral disturbances in pediatric patients. Increased susceptibility to cyclopentolate has been reported in infants, young children, and in children with spastic paralysis or brain damage. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place, and failure to recognize people. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld for four (4) hours after examination. Observe infants closely for at least 30 minutes (see WARNINGS).
-
ADVERSE REACTIONS:
Ocular: Increased intraocular pressure, burning, photophobia, blurred vision, irritation, hyperemia, conjunctivitis, blepharoconjunctivitis, punctate keratitis, synechiae have been reported. Non-ocular: Use of cyclopentolate has been associated with psychotic reactions and behavioral disturbances, usually in children, especially with 2% concentration. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place, and failure to recognize people. This drug produces reactions similar to those of other anticholinergic drugs, but the central nervous system manifestations as noted above are more common. Other manifestations of anticholinergic drugs are skin rash, abdominal distention in infants, unusual drowsiness, tachycardia, hyperpyrexia, vasodilation, urinary retention, diminished gastrointestinal motility and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Severe manifestations of toxicity include coma, medullary paralysis and death.
-
OVERDOSAGE:
Excessive dosage may produce behavioral disturbances, tachycardia, hyperpyrexia, hypertension, elevated intraocular pressure, vasodilation, urinary retention, diminished gastrointestinal motility and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Patients exhibiting signs of overdosage should receive supportive care and monitoring.
-
DOSAGE AND ADMINISTRATION:
Adults: Instill one or two drops of 1% or 2% solution in the eye which may be repeated in five to ten minutes if necessary. Complete recovery usually occurs in 24 hours. Complete recovery from mydriasis in some individuals may require several days. Children: Instill one or two drops of 1% or 2% solution in the eye which may be repeated five to ten minutes later by a second application of 1% solution if necessary.
-
HOW SUPPLIED:
Cyclopentolate Hydrochloride Ophthalmic Solution, USP is a sterile ophthalmic solution supplied in white opaque plastic dropper bottles as follows:
Cyclopentolate Hydrochloride Ophthalmic Solution USP, 1%
2 mL NDC: 17478-100-02
5 mL NDC: 17478-100-10
15 mL NDC: 17478-100-12Cyclopentolate Hydrochloride Ophthalmic Solution USP, 2%
2 mL NDC: 17478-097-02
5 mL NDC: 17478-097-10
15 mL NDC: 17478-097-12DO NOT USE IF IMPRINTED SEAL IS BROKEN OR MISSING.
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
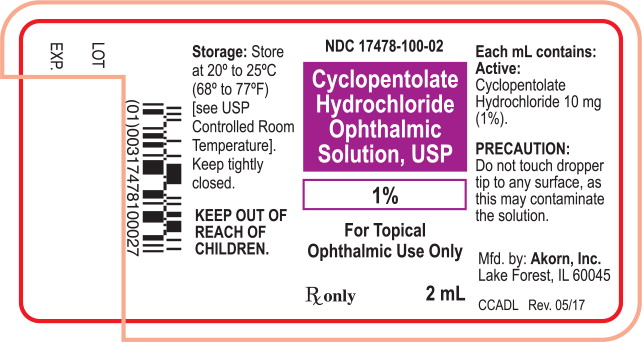
NDC: 17478-100-02
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
1%
For Topical
Ophthalmic Use Only
Rx only 2 mL
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
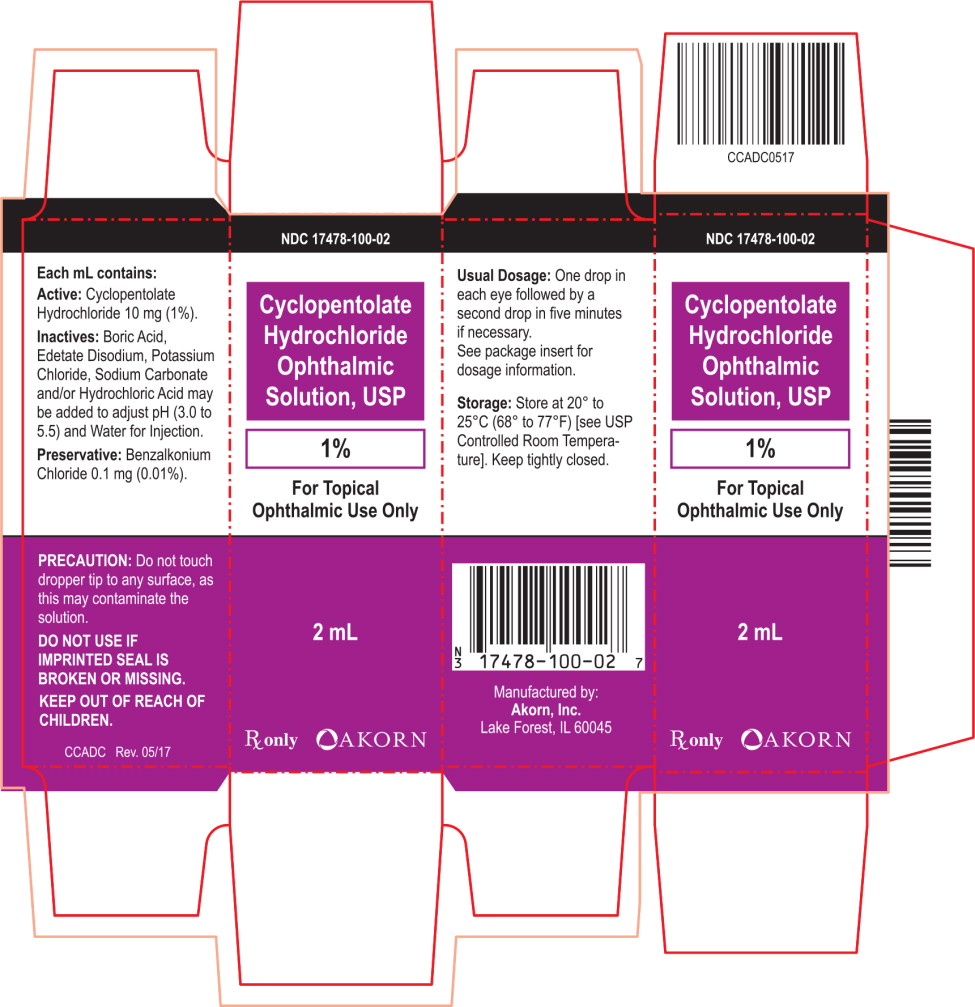
NDC: 17478-100-02
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
1%
For Topical
Ophthalmic Use Only
2 mL
Rx only Akorn logo
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
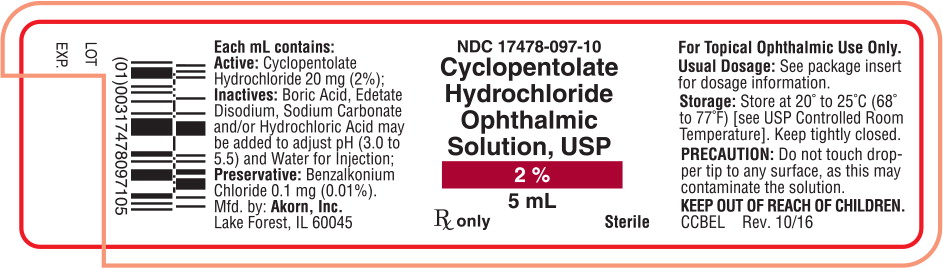
NDC: 17478-097-10
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
2%
5 mL
Rx only Sterile
-
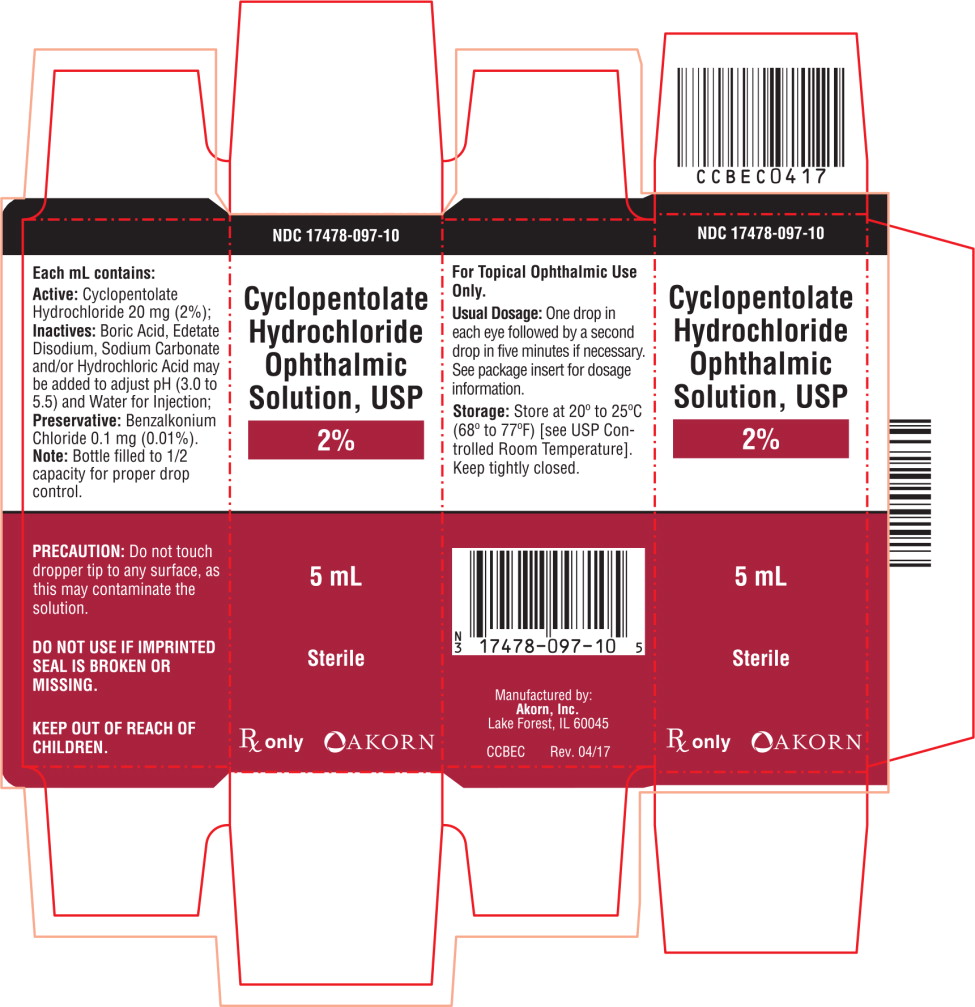
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-097-10
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
2%
5 mL
Sterile
Rx only Akorn logo
-
INGREDIENTS AND APPEARANCE
CYCLOPENTOLATE HYDROCHLORIDE
cyclopentolate hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cyclopentolate Hydrochloride (UNII: 736I6971TE) (Cyclopentolate - UNII:I76F4SHP7J) Cyclopentolate Hydrochloride 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) boric acid (UNII: R57ZHV85D4) edetate disodium (UNII: 7FLD91C86K) potassium chloride (UNII: 660YQ98I10) sodium carbonate (UNII: 45P3261C7T) hydrochloric acid (UNII: QTT17582CB) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-100-02 1 in 1 CARTON 01/13/1997 1 2 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 17478-100-10 1 in 1 CARTON 01/13/1997 2 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC: 17478-100-12 1 in 1 CARTON 01/13/1997 3 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040164 01/13/1997 CYCLOPENTOLATE HYDROCHLORIDE
cyclopentolate hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-097 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cyclopentolate Hydrochloride (UNII: 736I6971TE) (Cyclopentolate - UNII:I76F4SHP7J) Cyclopentolate Hydrochloride 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) boric acid (UNII: R57ZHV85D4) edetate disodium (UNII: 7FLD91C86K) sodium carbonate (UNII: 45P3261C7T) hydrochloric acid (UNII: QTT17582CB) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-097-02 1 in 1 CARTON 01/13/1997 1 2 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 17478-097-10 1 in 1 CARTON 01/13/1997 2 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC: 17478-097-12 1 in 1 CARTON 01/13/1997 3 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040165 01/13/1997 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE(17478-100, 17478-097) , REPACK(17478-100, 17478-097) , ANALYSIS(17478-100, 17478-097)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.