ARNICA- arnica montana tablet
ARNICA by
Drug Labeling and Warnings
ARNICA by is a Homeopathic medication manufactured, distributed, or labeled by Boiron, Boiron Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
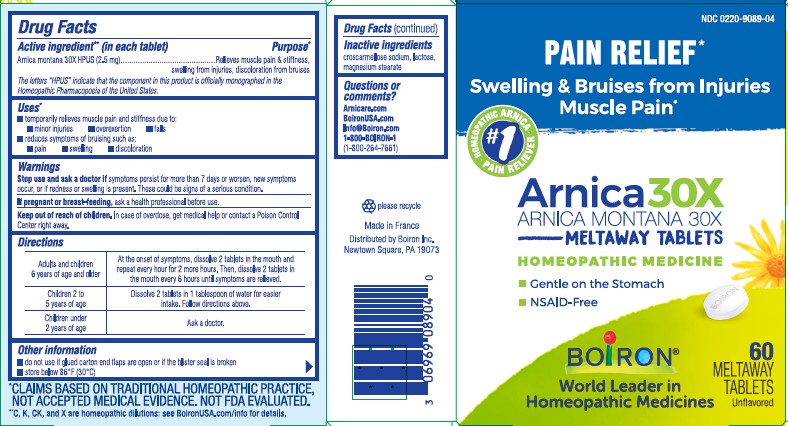
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Adults and children 6 years for age and older - At the onset of symptoms, dissolve 2 tablets in the mouth and repeat every hour for 2 more hours. Then, dissolve 2 tablets in the mouth every 6 hours until symptoms are relieved.
Children 2 to 5 years of age: Dissolve 2 tablets in 1 tablespoon of water for easier intake. Follow directions above.
Children under 2 years of age: Ask a doctor.
- INACTIVE INGREDIENT
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
do not use if glued carton ends flaps are open or if the blister seal is broken
store below 86°F (30°C)
PAIN RELIEF*
Swelling & Bruises from Injuries Muscle Pain*
60 Meltaway Tablets, unflavored
Gentle on the stomach
NSAID-Free
No Known Drug Interactions
Non-Drowsy
Meltaway Tablets Require No Food or Water
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICA
arnica montana tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0220-9089 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_X] Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) Product Characteristics Color white Score score with uneven pieces Shape ROUND (Boiron) Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0220-9089-04 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/01/2023 Labeler - Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9089)
Trademark Results [ARNICA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ARNICA 87317515 not registered Dead/Abandoned |
NICOLE STERN 2017-01-30 |
 ARNICA 77399483 3563474 Dead/Cancelled |
SING, KENNY 2008-02-18 |
 ARNICA 75088650 2184529 Dead/Cancelled |
Lozhkin, Igor 1996-04-11 |
 ARNICA 73366355 not registered Dead/Abandoned |
ARNICA INTERNATIONAL S. A. 1982-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.