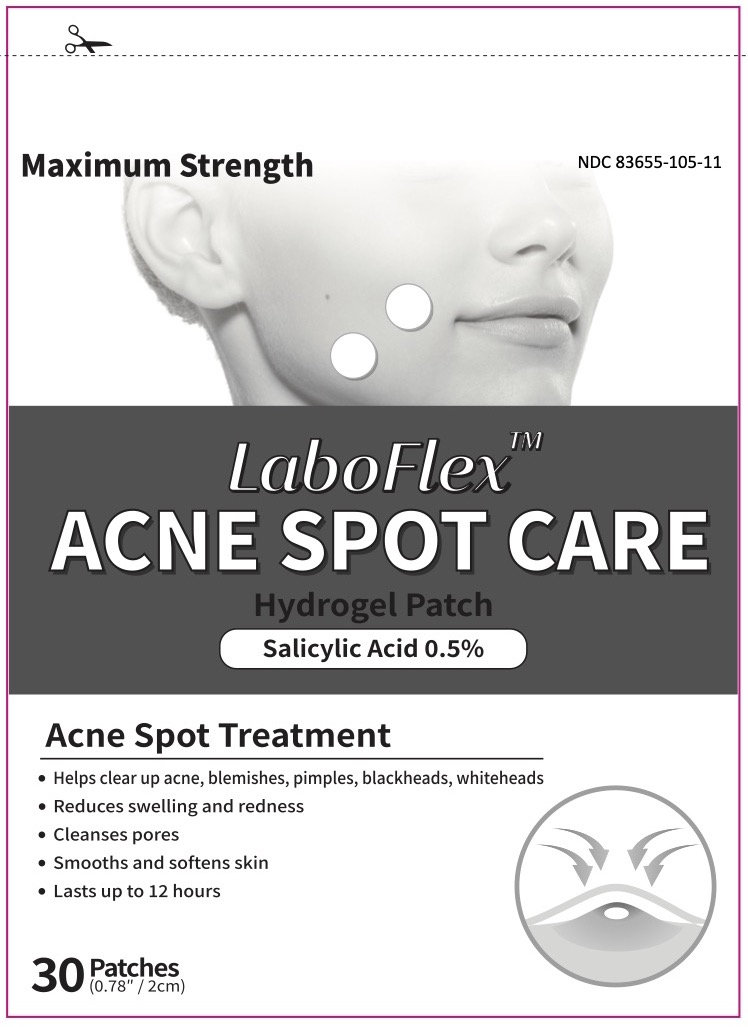
LABOFLEX Acne Spot Care by Laboflex, Inc. / WOOSHIN LAPACHE d.o.o. LABOFLEX Acne Spot Care
LABOFLEX Acne Spot Care by
Drug Labeling and Warnings
LABOFLEX Acne Spot Care by is a Otc medication manufactured, distributed, or labeled by Laboflex, Inc., WOOSHIN LAPACHE d.o.o.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LABOFLEX ACNE SPOT CARE- salicylic acid 0.5% patch
Laboflex, Inc.
----------
LABOFLEX Acne Spot Care
Directions
- Cleanse thoroughly before applying this product
- Cover the entire affected area with patch
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or even every other day
Inactive Ingredients
Carboxymethylcellulose Sodium, Dihydroxyaluminum Aminoacetate, Edetate Sodium, Glycerin, Methyl- paraben, Mint Oil, Partially Neutralized Polyacrylic Acid, Polysorbate 80, Povidone-K90, Propylparaben, Propylene Glycol, Sodium Polyacrylate, Tartaric Acid, Titanium Dioxide, Purified Water
| LABOFLEX ACNE SPOT CARE
salicylic acid 0.5% patch |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Laboflex, Inc. (128394050) |
| Registrant - Laboflex, Inc. (128394050) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| WOOSHIN LAPACHE d.o.o. | 507385209 | manufacture(83655-105) | |
Revised: 12/2024
Document Id: 28548382-cb72-9bf5-e063-6394a90ad98a
Set id: 06987b0d-a8f7-82e7-e063-6394a90aa7e1
Version: 2
Effective Time: 20241202
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.